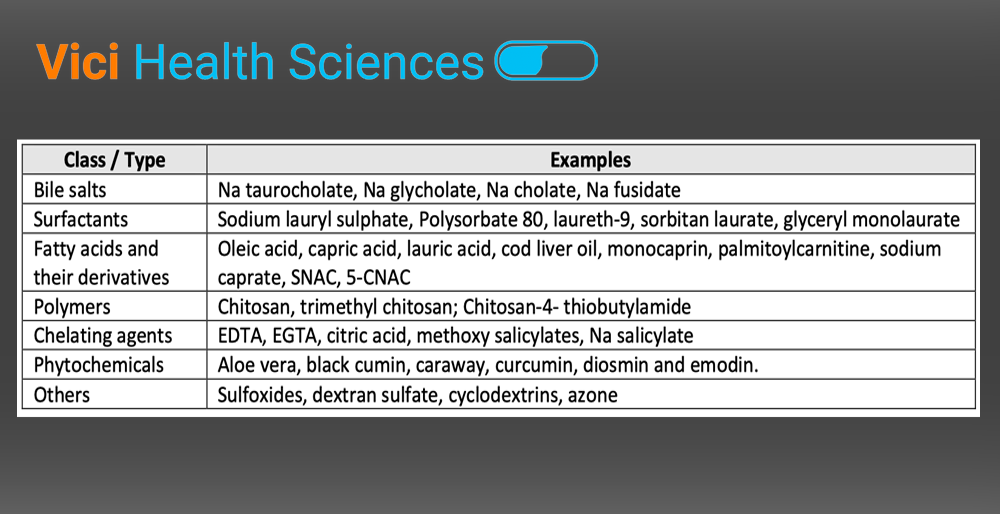
by Anish Dhanarajan | May 17, 2024 | Blog
Numerous potential compounds in pharmaceutical development are categorized under BCS classification III and IV. These compounds exhibit poor bioavailability due to their low permeability across biological membranes. These includes hydrophilic small molecules, which do...
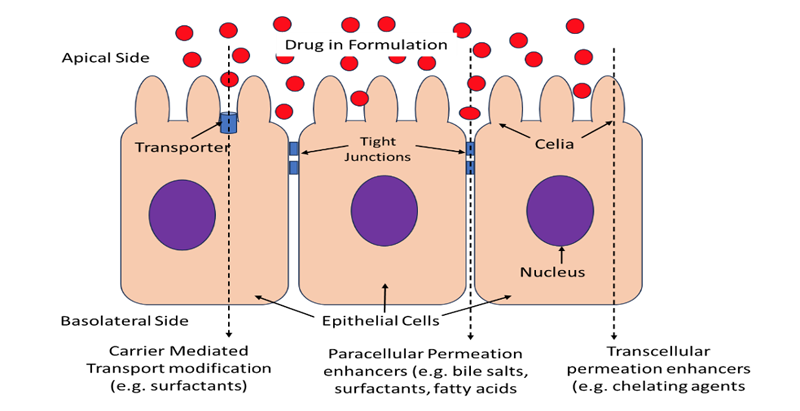
by Anish Dhanarajan | May 15, 2024 | Blog
A large number of promising compounds in the pharmaceutical pipelines fall under BCS classification III and IV that show poor bioavailability due to low permeability across biological membranes. Often the only option for safe and effective delivery for these...
by francesca@vicihealth.com | Apr 22, 2024 | Blog
Muco-Adhesion and Muco-adhesive Delivery Systems Muco-adhesion refers to the adhesion between two materials, one of which is a mucus layer. Mucoadhesive delivery systems can be administered through a variety of routes including oral, nasal, ocular, rectal, vaginal,...
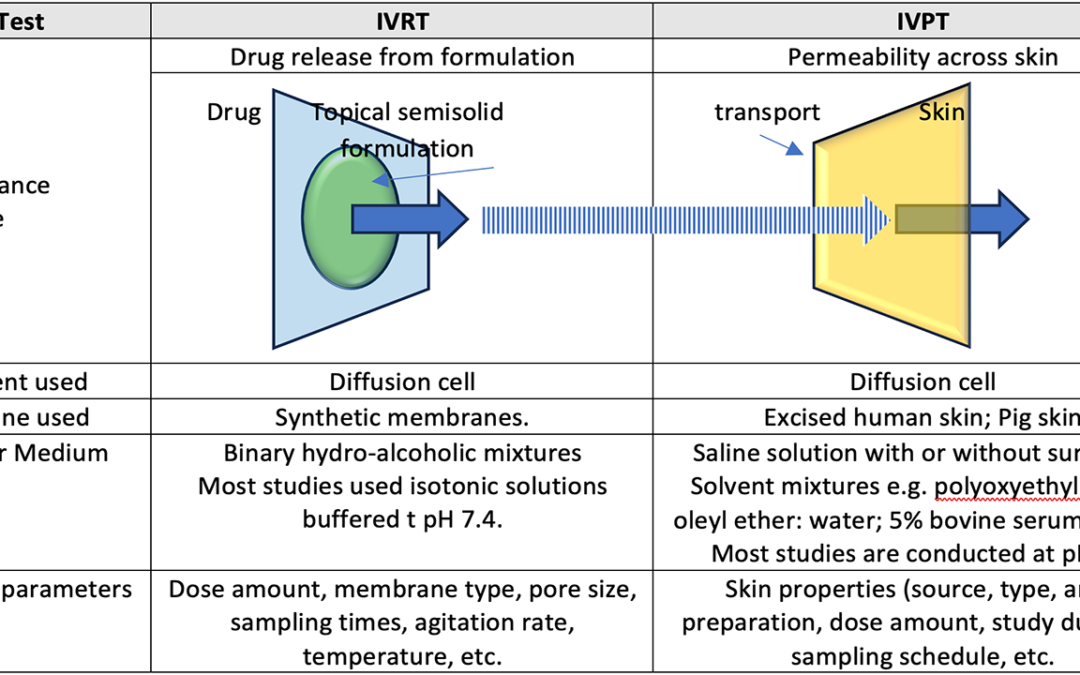
by francesca@vicihealth.com | Apr 16, 2024 | Blog
Topical products are liquid or semi-solid dosage forms applied to the skin, encompassing both integumentary and mucosal membranes. They can be developed as liquids, gels, creams, ointments and lotions. Semisolid dosage forms can be viewed as extended-release...

by Alex Brown | Mar 15, 2024 | Blog
Topical dosage forms are medications applied directly to the body surfaces, primarily the skin or mucous membranes. Topical products are typically liquid or semi-solid dosage forms that are widely used due to ease of application. There are many advantages of topical...

by Alex Brown | Sep 18, 2023 | Blog
In the ever-evolving landscape of pharmaceuticals and drug discovery, researchers are constantly on the lookout for innovative compounds that can target diseases more effectively with fewer side effects. One class of compounds that continues to be critical in this...







