BLOG
Non-Clinical (animal) IND Enabling Studies: What You…
In drug development, IND enabling studies serve as a vital…
Key Pharmacokinetic Factors in Topical Medication Development
Topical drug products are designed to deliver active ingredient on…
Clinical Manufacturing Explained: How CDMOs Support the…
The development of clinical trial material (CTM) is a critical…
NDA vs. ANDA: A Comprehensive Guide to…
A New Drug Application (NDA) and an Abbreviated New Drug…
Stability Chambers: Testing Methods, Essential Equipment, and…
Stability chambers are a must-have in pharmaceutical research and development.…
Top 7 Questions for Small Pharma and…
Most pharma companies use contract development and manufacturing organizations (CDMOs)…
Semi-Solid and Semi-Liquid Dosage Forms
Semi-solid and semi-liquid dosage forms are key pharmaceutical preparations that…
The Critical Role of Formulation Development in…
All medicine administered to patients must first be formulated. It’s…
The FDA’s 505(b)(2) Explained
Pharmaceutical innovation can stem from a variety of different facets…
What is a CDMO and Why Does…
What is a CDMO? A CDMO, also sometimes referred to…
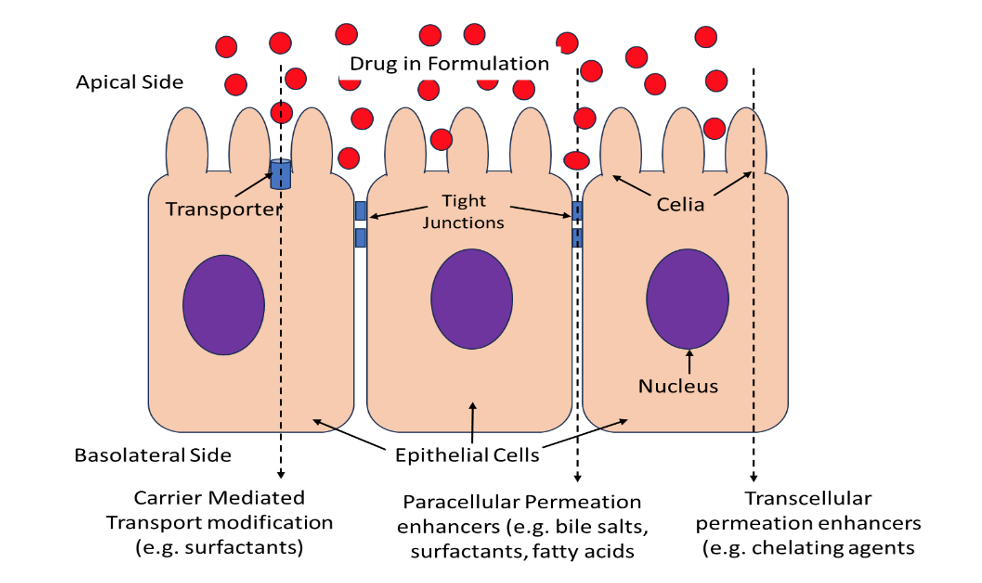
Permeability Enhancers for Bioavailability Improvement
A large number of promising compounds in the pharmaceutical pipelines…
Muco-Adhesion Testing
Muco-Adhesion and Muco-adhesive Delivery Systems Muco-adhesion refers to the adhesion…
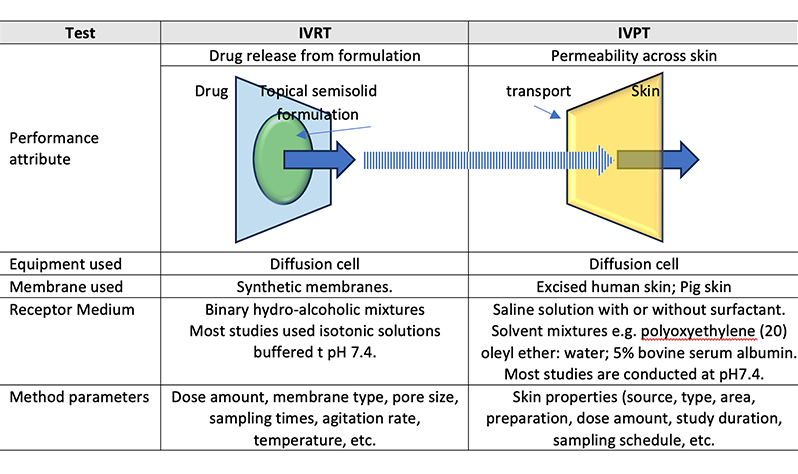
IVRT and IVPT: Pivotal Tools in Topical…
Topical products are liquid or semi-solid dosage forms applied to…
Topical Dosage Forms: Product Development Overview
Topical dosage forms are medications applied directly to the body…
Heterocyclic Compounds, the Backbone of Small Molecule…
In the ever-evolving landscape of pharmaceuticals and drug discovery, researchers…
Pharmaceutical Suspensions – Part 2: Formulation Development
Early Preformulation Studies For the development of a robust suspension…
Pharmaceutical Suspension Formulations – Part 1: An…
What are Pharmaceutical Suspensions Liquid oral dosage forms such as…
Buccal Delivery Systems (Part 3) – Improving…
Poor bioavailability is a common problem for a majority of…
Buccal Delivery Systems | Part 2: Applications…
Why Buccal Delivery Buccal cavity in the mouth is an…
Buccal Delivery Systems | Part 1: Opportunities…
The buccal region is the mouth cavity offers unique benefits…