Topical products are liquid or semi-solid dosage forms applied to the skin, encompassing both integumentary and mucosal membranes. They can be developed as liquids, gels, creams, ointments and lotions. Semisolid dosage forms can be viewed as extended-release preparations, with the rate of drug release influenced by the formulation and manufacturing process.
Evaluation of Topical products
Topical products are characterized using– Product quality tests and product performance tests. Product quality tests are used to assess the physical, chemical and microbiological properties. Product performance testing evaluates the attributes influencing the release and permeation of drug from the dosage form through skin.
Two critical tools in the development and evaluation of topical products are In Vitro Release Testing (IVRT) and In Vitro Permeation Testing (IVPT). IVRT test measures of drug release rate from the formulation whereas IVPT studies assess the rate and extent of drug permeation through excised human skin. While these product performance tests are not expected to predict the therapeutic effect of the drug, they can identify in vitro modifications due to meaningful / intentional variations in formulation and manufacturing parameters. These alterations include properties of the drug substance and excipients, formulation composition, structure and physical properties, manufacturing process, shipping and storage effects.
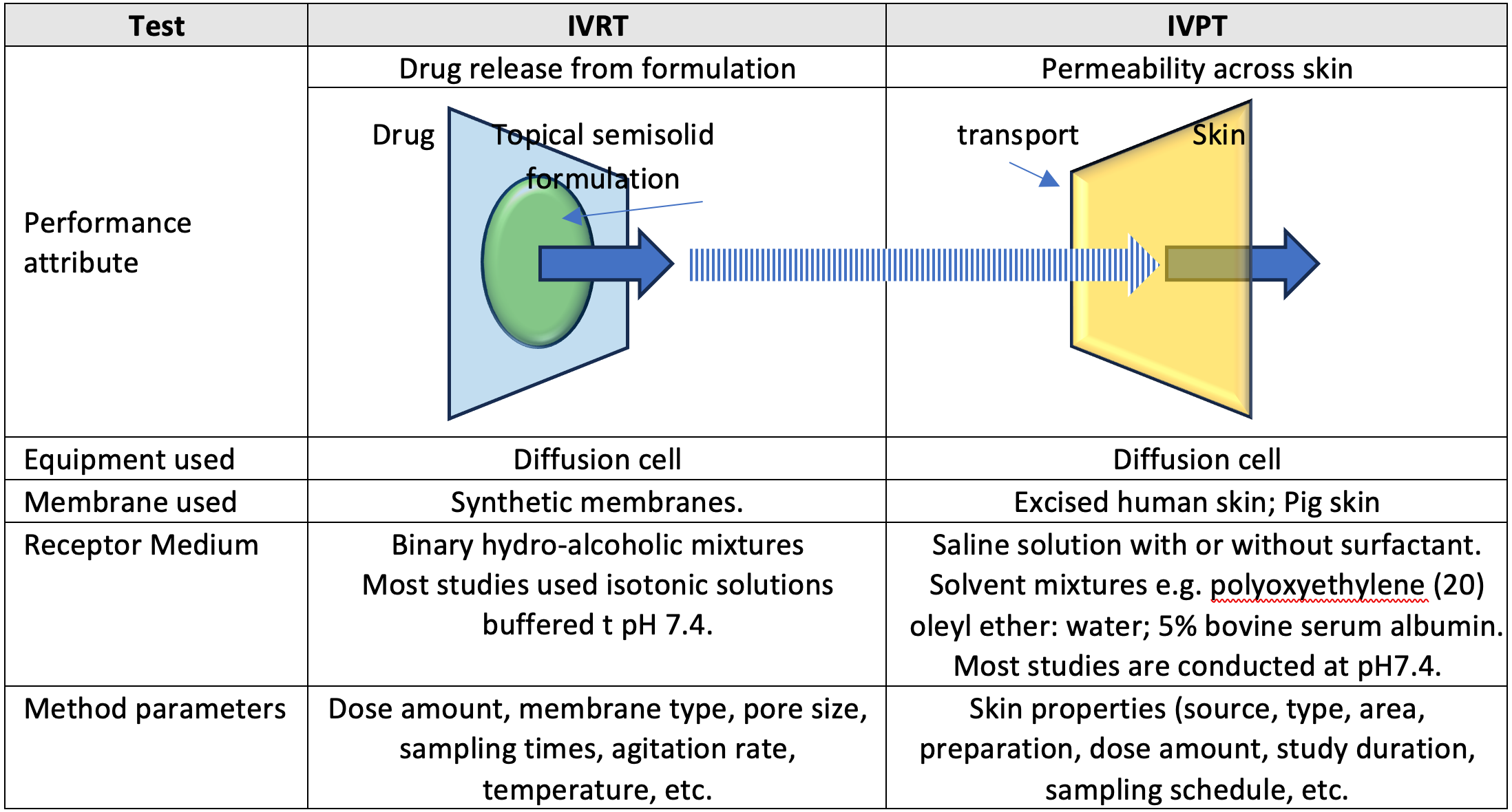
In-Vitro Release Testing
IVRT is used to measure the release rate of drug from a topical formulation. It provides valuable information about the consistency and reliability of the drug release process, which is crucial for ensuring the effectiveness of the product. IVRT is typically performed using a diffusion cell apparatus (e.g. Franz diffusion cell), where the formulation is applied to a non-interactive synthetic membrane and the amount of drug released over time is measured. An adequate IVRT method will discriminate and detect any product changes that may impact performance.
In-Vitro Permeation Testing
IVPT, is a more complex test that simulates the permeation of drug through skin. It provides a closer approximation to the in vivo performance. IVPT is performed using a setup similar to IVRT, but with the addition of a biological membrane (usually excised human or animal skin). The flux profiles in IVPT studies are similar to pharmacokinetic profiles and can be examined using specific IVPT endpoints. These endpoints are somewhat comparable to the pharmacokinetic endpoints of maximum concentration (Cmax) and the area under the concentration-time curve (AUC).
Applications of IVRT/IVPT
For any dosage form, the drug release is a critical parameter utilized throughout the various phases of product development. During development of topical semi-solid products, IVRT and IVPT play a crucial role in determining the rate of drug release and diffusion
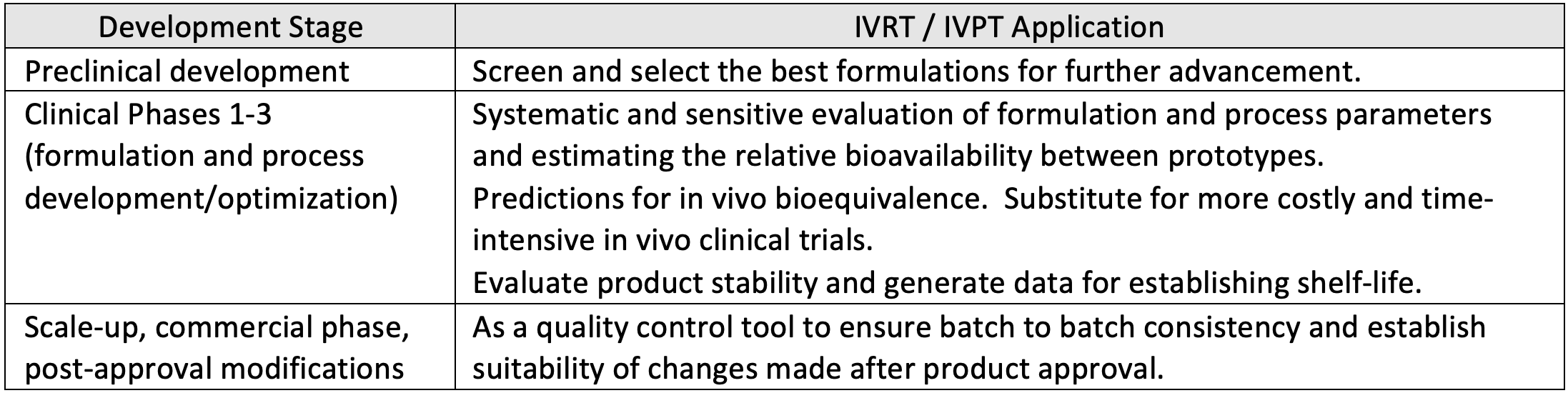
In case of topical generic products, FDA has released guidances outlining the recommendations for conducting IVRT and IVPT studies to compare generic product with the Reference Listed Drug (RLD) with the objective to support demonstration of bioequivalence. FDA has also published several product specific guidances that provide options to use IVRT and IVPT tests (in conjunction with Q1/Q2/Q3 similarity demonstration) as an alternative to complex, costly and time-consuming in-vivo clinical trials.
Vici Health Sciences – Capabilities and Services
Vici Health Sciences is equipped with deep knowledge, experience and equipment for developing semi-solid dosage forms intended for topical use. We use in-vitro performance testing along with physicochemical, rheological and structural characterization to facilitate the rapid development of these products. These capabilities coupled with our knowledge on regulatory aspects of IVPT and IVRT makes us an ideal CDMO partner for designing and conducting these studies.
