A large number of promising compounds in the pharmaceutical pipelines fall under BCS classification III and IV that show poor bioavailability due to low permeability across biological membranes. Often the only option for safe and effective delivery for these compounds is through injectable routes. However, it is desirable to develop non-injection formulations due to numerous reasons such as increased convenience, patient compliance, decreased variability in systemic exposure, reduced dose thus resulting in reduced side effects and cost of API. Low drug permeability poses considerable challenge to the development of bioavailable and reproducible dosage forms intended for more desirable routes such as oral, nasal, buccal, sublingual, transdermal, vaginal or rectal administration. To overcome these, the use of chemical permeability enhancing excipients has been one of the simplest and prominent formulation approaches to enable non-injection delivery.
Permeability Enhancers
Permeability enhancers, also known as absorption enhancers, are substances that facilitate drug transport across biological membranes. They work by transiently altering the physicochemical properties of the membrane, thereby increasing its permeability to drugs. These excipients enable increased systemic exposure after oral, transmucosal, or transdermal administration as demonstrated by enhanced bioavailability and improved therapeutic outcomes. Thus, they provide viable alternatives to current products that provide sub-optimal bioavailability or require injection-based administration.
Mechanism of Permeability Enhancement
In order for a drug to penetrate through biological membranes, it must overcome several natural barriers including poor transcellular permeability, tight junctions, metabolism, pH, ciliary movement and transporters. Permeability enhancers can increase membrane permeability through several mechanisms such as modification of partitioning coefficient, interacting with lipid bilayer of membranes, fluidization or disordering of lipids, changing membrane integrity thereby increasing transcellular membrane permeability. Other enhancers act by modifying the tight junctions between cells, allowing for paracellular transport of drugs or by affecting the function of membrane transporters.
Types of Permeability Enhancers
Permeability enhancers can be broadly classified into several categories. Bile salts, surfactants, fatty acids, and certain polymers work by disrupting the structure of the lipid bilayer of the membrane. Others such as chelating agents can open the tight junctions between cells. Certain surfactants such as sodium lauryl sulfate inhibit secretory (efflux) drug transporters on the apical surface. Certain fatty acids such as sodium caprate act both by dilating the tight junction (paracellular permeation) as well as increasing transcellular penetration.
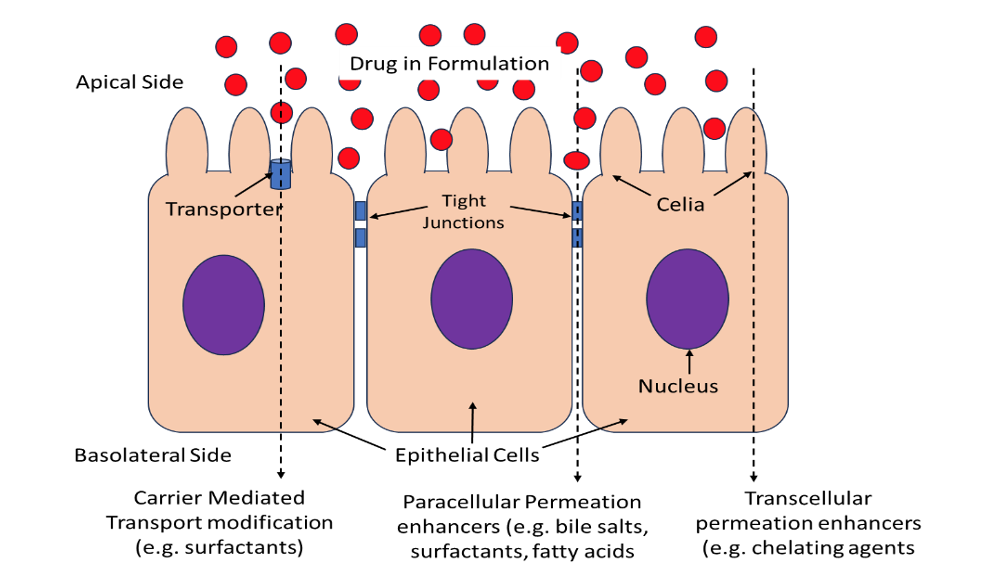
Applications
Permeability enhancers have found wide applications in oral, transmucosal and transdermal drug delivery. They are commonly used in oral formulations of BCS class II and IV drugs which are often hydrophilic small molecules with insufficient partitioning into biological membranes. An important emerging application is in oral formulations of peptides and proteins, which are typically poorly absorbed from the gastrointestinal tract due to their large size and instability. These benefits have enriched the pharmaceutical R&D pipelines by enabling a wider range of low permeability compounds. Opportunities also exist for lifecycle management of marketed products that are known to exhibit poor and variable efficacy due to poor drug permeability.
Effect of permeability enhancers can be further enhanced by using them in conjunction with other technologies such as bio-adhesion, gastro-retention, iontophoresis, enteric encapsulation, targeting the drug in the site of maximum absorption of GIT, use of protease inhibitors, inhibition of secretory transport mechanisms, pH modification, complexation, carrier and ion pairing.
Analytical Techniques
Typically, during early phase development, potential permeability enhancers are screened using validated and standardized in-vitro permeability methods using Caco2 cells monolayers. In-situ animal intestinal perfusion studies can also be used to assess permeability of the drug through the membrane targeted as the delivery route. More recently, in-vitro release test and in-vitro permeability tests are also used to evaluate topical semisolid and liquid dosage forms.
Vici Health Sciences – Capabilities and Services
Permeability enhancers play a crucial role in increasing the bioavailability of low permeability drugs. However, their use must be carefully considered and thoroughly evaluated to ensure stability, safety and efficacy. Vici Health Sciences has extensive experience and knowledge in use of permeability enhancers for dosage forms for both small and large molecule drugs. This makes us an ideal CDMO partner for product development, analytical and regulatory support.
