
by Alex Brown | Sep 14, 2023 | Blog
Early Preformulation Studies For the development of a robust suspension product, several factors must be considered early on during product development. These include API particle size, particle density, viscosity and specific gravity of the media, buffer capacity,...

by Alex Brown | Aug 29, 2023 | Blog
What are Pharmaceutical Suspensions Liquid oral dosage forms such as solutions and suspensions are often preferred for pediatric or geriatric patients that find it difficult to swallow tablets or capsules. Most pharmaceutical suspensions are coarse dispersions of...
by Alex Brown | Aug 4, 2023 | Blog
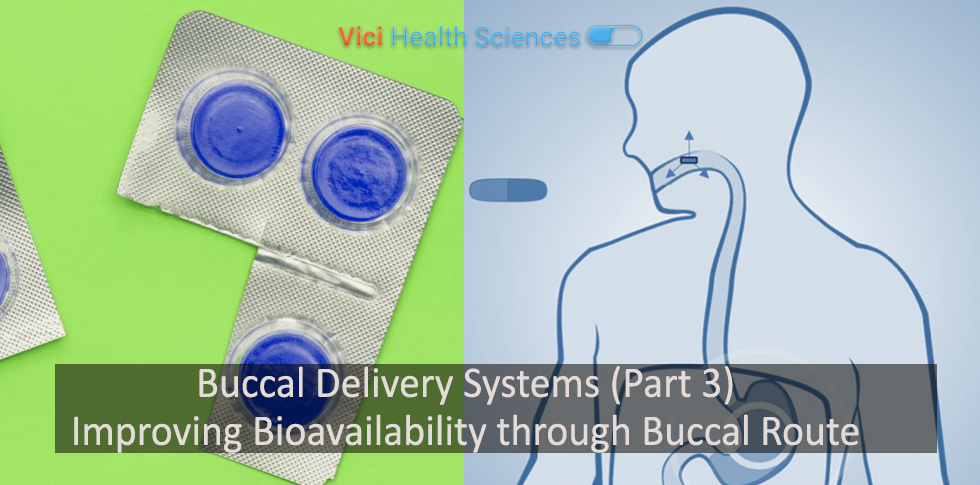
Poor bioavailability is a common problem for a majority of new drugs in the pipeline of pharmaceutical companies. This can be due to a variety of reasons including poor solubility, permeability, susceptibility to low pH environment, enzymatic degradation or hepatic...
by Alex Brown | Jul 13, 2023 | Blog
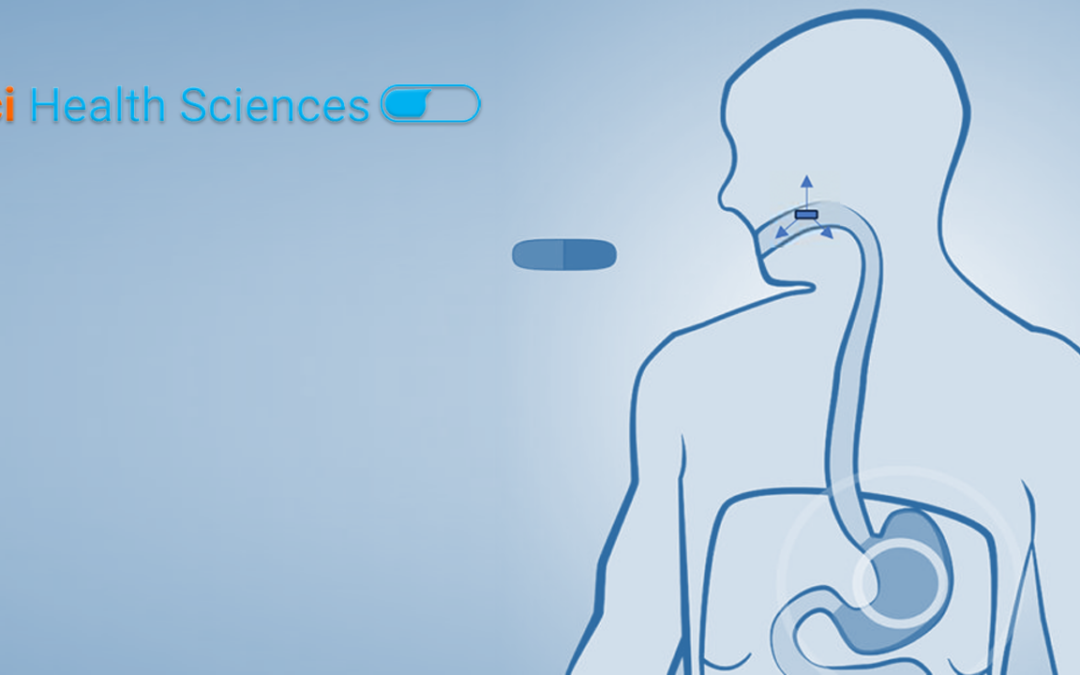
Why Buccal Delivery Buccal cavity in the mouth is an attractive route for administration of drugs that offers numerous benefits such as rapid product development timelines, reduced cost of therapy, ease of administration and improved adherence to therapy. It is...
by Alex Brown | Jul 12, 2023 | Blog
The buccal region is the mouth cavity offers unique benefits for delivery of drugs over conventional more popular routes of administration such as oral, parenteral and topical. Buccal mucosa is highly perfused with blood vessels which enables rapid absorption and...

by Alex Brown | Apr 20, 2023 | Blog
Taste masking is often used in the pharmaceutical industry to make bitter or unpleasant-tasting drugs more palatable. This is especially important for pediatric populations or geriatric populations. Taste masking can be achieved through a variety of methods,...







