Will the US FDA accept foreign clinical trials?

In the past, the FDA only accepted clinical studies that were conducted in the United States. However, in recent years, the FDA has begun to accept clinical studies that were conducted in other countries. This is because the FDA recognizes that high-quality clinical studies can be conducted in other countries, and that accepting such data […]
FDA approval pathways: the 505b2

Pharmaceutical innovation stems from a variety of sources. Practicing doctors and healthcare providers generate concepts and collect observational data for new and improved treatments using existing approved medication. Alternatively, university researchers study existing molecules for different disease models in vitro or in vivo. Pharmaceutical companies license these assets and undertake preclinical and clinical development with […]
Vici can now export and import DEA schedule 1-5 controlled substances to support clinical development

Vici Health Sciences, a customer-focused pharmaceutical CDMO that offers formulation development services, R&D program management, FDA regulatory support, phase 1 and phase 2 cGMP clinical batch manufacturing has added DEA scheduled 1-5 controlled substances import and export capability. The expansion in capability was made to support sponsors with clinical development needs for both NDA and […]
Nitrosamine risk evaluation and testing, what’s next?

Nitrosamine compounds have long been considered probable human carcinogens. Nitrosamines are produced by the reaction of nitrites and secondary amines. Nitrosamine compounds are ubiquitous in food, beverages, and the environment. In 2018, angiotensin II receptor blockers (ARB) medicines were withdrawn from the market in the US due to the presence of an impurity, N-nitrosodimethylamine (NDMA). […]
Creating Formulation Intellectual Property during Drug Development – FAQ

Why must companies consider formulation patents? Pharmaceutical drug development is a costly endeavor. It is critical that pharmaceutical companies secure return on investment in their innovation. Valuation of smaller, pre-revenue companies often depends on the quantity and quality of relevant intellectual property (IP) they own. Formulation development requires innovation and problem-solving, resulting in surprising results […]
CMO versus CDMO: what’s the difference and how this decision is make or break for early phase drug development programs

Choosing the right organization to develop drugs is the single most important decision a small or mid-size pharmaceutical company is faced with after selecting the molecule and indication. There are many different types of organizations to choose from. Some organizations are CMO’s (contract manufacturing organizations) while others are CDMOs (contract development and manufacturing organizations). What’s […]
Focusing on medicine for patients with swallowing difficulties

Vici Health Sciences, a Maryland-based CDMO is pleased to introduce cGMP API and API intermediate synthesis services to support phase 1 first-in-human clinical studies and GLP nonclinical studies. This program is built on Vici’s deep expertise in CMC (Chemistry Manufacturing and Controls) ranging from early-phase API and intermediate synthesis through IND enabling cGMP clinical supplies […]
Vici now offers cGMP API and intermediates synthesis services to support phase 1 clinical and GLP nonclinical studies
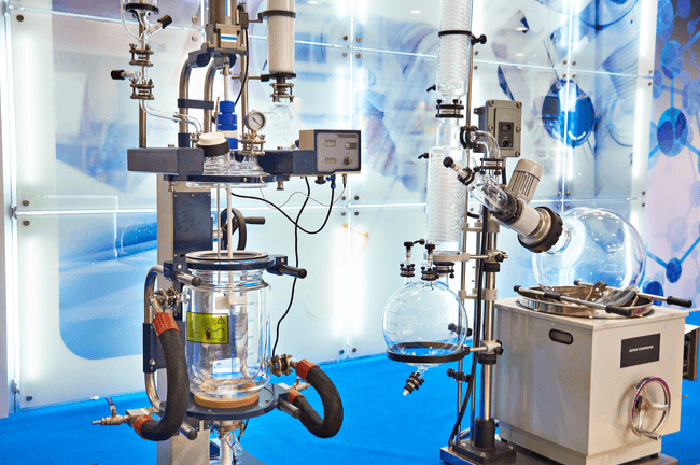
Vici Health Sciences, a Maryland-based CDMO is pleased to introduce cGMP API and API intermediate synthesis services to support phase 1 first-in-human clinical studies and GLP nonclinical studies. This program is built on Vici’s deep expertise in CMC (Chemistry Manufacturing and Controls) ranging from early-phase API and intermediate synthesis through IND enabling cGMP clinical supplies […]
High quality formulation development upfront saves drug developers valuable time and money
Drug development leaders must consider robustness and quality initially during formulation development. In the past, pharmaceutical researchers often did not have a common R&D quality framework for developing robust formulations and manufacturing processes. Consequently, quality was ensured through QC analytical testing. However, in cGMP manufacturing, this leads to expensive batch failure and troubleshooting resulting in […]
The Importance of Formulation Development in Successful Drug Development

Formulation development is a critical part of any drug development program. Once a sponsor selects a new chemical entity (NCE) or 505(b)(2) drug candidate for first-in-human Phase 1 clinic study or a Phase 2 efficacy study, the focus must be on selecting a strong formulation development team to complete a successful investigational new drug (IND) application. Many critical decisions are made […]

