CDMO vs CMO vs CRO: Key Differences

In the pharmaceutical industry, CROs refer to contract research organizations that provide clinical trial services and non-clinical toxicology services to clients. On the other hand, CMOs and CDMOs refer to Contract Manufacturing Organizations and Contract Development and Manufacturing Organizations that primarily provide drug manufacturing and drug development services. For small or mid-sized pharma companies looking […]
Obstacles in Drug Development and How to Overcome Them

Drug discovery and development is a complex, expensive undertaking that can often end in failure to secure drug approval for critically needed medicines. The pre-clinical and clinical phase of drug development starts after target identification. During this phase, missteps in overall strategy, IND-enabling animal studies, lack of bioavailability or drug stability, formulation and manufacturing challenges, […]
Vici Health Exhibiting at 23rd Annual Contract Pharma in New Brunswick, NJ

Vici Health Sciences is exhibiting at the 23rd Annual Contract Pharma Conference on September 26-27 at the Hyatt Regency, New Brunswick, NJ. Our founder and CEO, Dr. Anish Dhanarajan, will be in attendance to discuss and answer questions on how Vici can provide high quality, cost-effective drug development solutions for your pharmaceutical programs. Visit us […]
Vici Health Sciences Attending 2024 Chem Outsourcing Conference in Parsippany, NJ

Vici Health Sciences will be attending the 2024 Chem Outsourcing Conference on Thursday, September 5th, 2024 in Parsippany, NJ. Date: September 5th from 7am-6pmLocation: Hilton ParsippanyAddress: 1 Hilton Ct, Parsippany, NJ 07054 This is a great opportunity to meet with our founder and CEO, Dr. Anish Dhanarajan, to discuss how Vici can provide formulation development, […]
Permeability Enhancers for Bioavailability Improvement
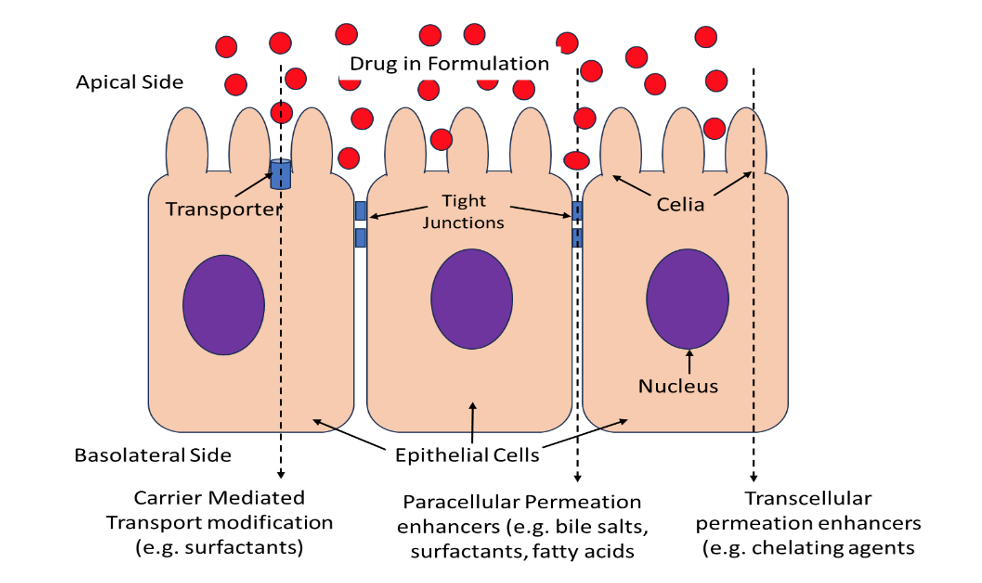
A large number of promising compounds in the pharmaceutical pipelines fall under BCS classification III and IV that show poor bioavailability due to low permeability across biological membranes. Often the only option for safe and effective delivery for these compounds is through injectable routes. However, it is desirable to develop non-injection formulations due to numerous […]
Muco-Adhesion Testing

Muco-Adhesion and Muco-adhesive Delivery Systems Muco-adhesion refers to the adhesion between two materials, one of which is a mucus layer. Mucoadhesive delivery systems can be administered through a variety of routes including oral, nasal, ocular, rectal, vaginal, sublingual and buccal. Besides ease of application, these systems provide unique benefits such providing direct access to systemic […]
IVRT and IVPT: Pivotal Tools in Topical Product Development
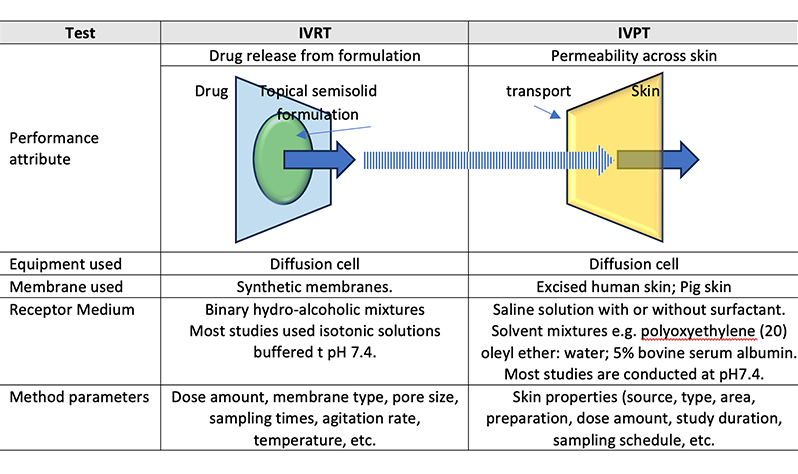
Topical products are liquid or semi-solid dosage forms applied to the skin, encompassing both integumentary and mucosal membranes. They can be developed as liquids, gels, creams, ointments and lotions. Semisolid dosage forms can be viewed as extended-release preparations, with the rate of drug release influenced by the formulation and manufacturing process. Evaluation of Topical products […]
Topical Dosage Forms: Product Development Overview

Topical dosage forms are medications applied directly to the body surfaces, primarily the skin or mucous membranes. Topical products are typically liquid or semi-solid dosage forms that are widely used due to ease of application. There are many advantages of topical route of delivery including elimination of hepatic first pass metabolism, drug deliver directly to […]
Meet with Vici at AAPS 2023 PharmSci 360

Are you looking for a high quality, US-based, full service R&D focused CDMO to accelerate your drug development program? Meet with our CEO, Dr Anish Dhanarajan and Senior Director of R&D, Dr Suneel Rastogi at AAPS next week to discuss your needs. Vici offers cGMP API synthesis for nonclinical and phase 1 clinical studies, formulation development services for a variety of […]
Heterocyclic Compounds, the Backbone of Small Molecule Therapeutics

In the ever-evolving landscape of pharmaceuticals and drug discovery, researchers are constantly on the lookout for innovative compounds that can target diseases more effectively with fewer side effects. One class of compounds that continues to be critical in this pursuit is heterocyclic molecules. These compounds are characterized by the presence of at least one ring […]

