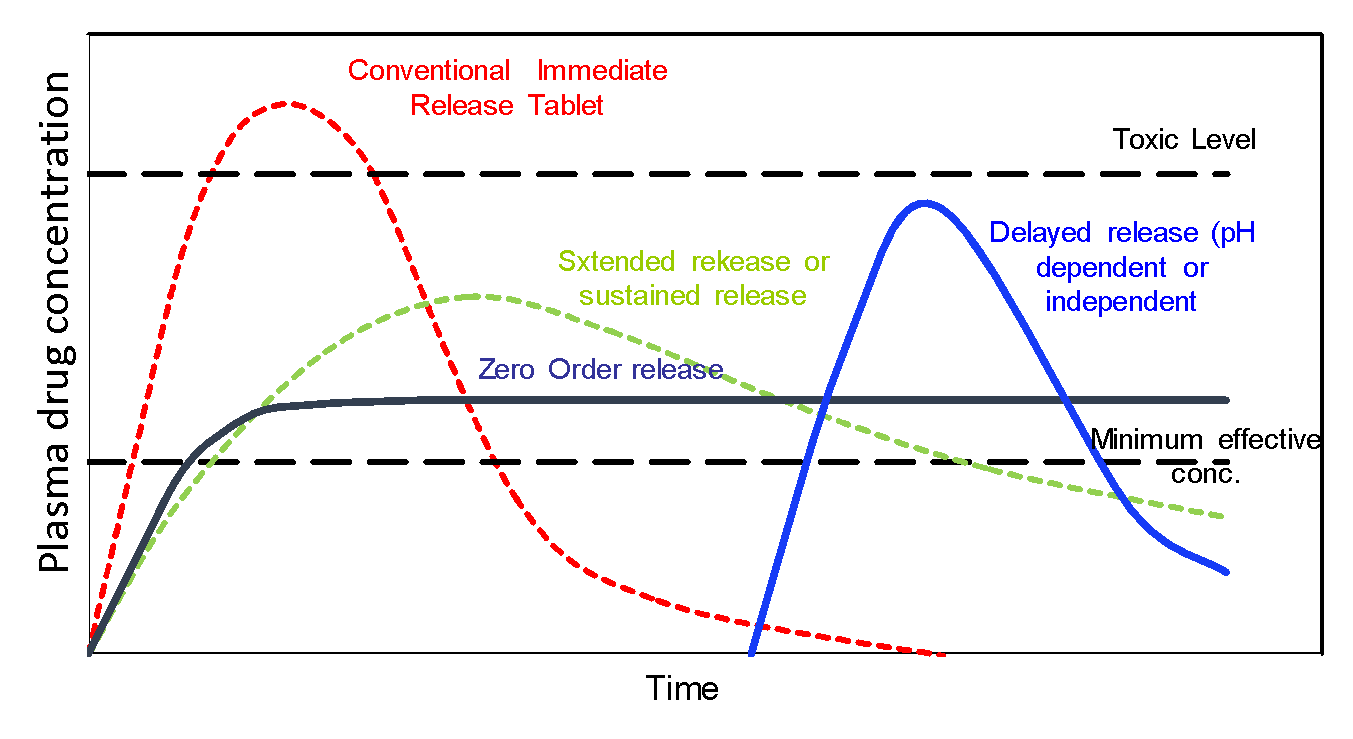
Controlled Release (CR) involves the application of one or more polymer system to design a drug product that controls the drug release in a desired well characterized and reproducible manner. This results in drug entry into the systemic circulation within the specifications of the required drug delivery profile. Figure below compares plasma drug profiles for conventional immediate release dosage forms with that of a variety of currently available controlled release technologies. Depending on the application, these technologies can be delivered through different routes of administrations such as oral, topical, intramuscular, subcutaneous and ophthalmic delivery.
Advantages of Controlled Release
Conventionally, product development of new molecular entities typically starts with immediate release (IR) dosage forms. These formulations provide rapid drug release and absorption which may not be optimal for product performance. Typically, there is a rapid and steep increase in plasma concentration followed by a decline that depends on drug elimination kinetics. Due to this, there is a very narrow window of time when the drug is effective but doesn’t show toxic side effects. Consequently, the drug must be administered frequently multiple time per day.
Controlled release products overcome many of the limitations of the IR dosage forms. The decreased frequency of administrations leads to decreased pill burden and better patient compliance. This is especially useful for chronic therapy. The peaks and troughs in plasma levels that are observed in IR dosage forms are minimized. This results in decreased severity or frequency of side effects, minimized chances of dose dumping, increased duration of effectiveness and thus overall better therapeutic outcomes. These factors are especially important for low therapeutic index drugs. Other unique advantages offered by controlled release products include site specific delivery and abuse deterrence. Besides clinical advantages, these technologies are used to create differentiation, innovation, intellectual property and life-cycle management opportunities.
Type of Controlled Release Dosage Forms
CR formulations are available in a verity of dosage forms such tablets, beads, capsules, suspensions, transdermal patches and long acting injectables. Based on the mechanism of release these products can be broadly categorized as follows:
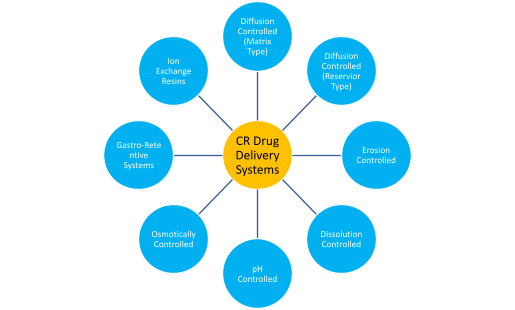
Diffusion controlled: In these systems, the drug release is modulated by diffusion of the drug through high molecular weight polymers. For such systems, the drug release rate is proportional to the surface area and solubility of drug but inversely proportional to the thickness of the diffusion layer. Two types of diffusion based controlled release formulations – matrix and reservoir are most commonly used for extended or sustained release applications.
- Matrix type: Here the active ingredient in imbedded in a matrix of hydrophilic polymer. When such an oral tablet is exposed to the gastrointestinal (GI) environment, the media diffuses into it. This causes the polymer to swell and hydrate. The drug then dissolves and diffuses out in the media and finally absorbed into the body. These matrix systems decrease the possibility of dose dumping thus avoiding serious adverse effects.
- Reservoir type: Here the drug is enclosed inside a coating layer of controlled release polymer. When the product is exposed to the GI environment, the media diffuses through the polymer layer into the product. The drug then dissolves and diffuses out through the polymer layer. Since the surface area and thickness of the coating layer is relatively unchanged during the dissolution process, these reservoir type systems can provide a more constant release of drug over time.
Erosion controlled: In these systems the drug is embedded in a matrix of hydrophobic insoluble polymer or a wax. When such a system is exposed to the aqueous medium of the GIT, the polymer matrix slowly erodes thus progressively exposing newer layers of drug for dissolution. Thus, the drug dissolution rate is controlled by the rate of erosion of tablet matrix.
Dissolution controlled: These formulations provide sustained release of poorly soluble drugs for a prolonged period. The release profile is controlled by altering the particle size hence the specific surface area of the drug. Example of such products include major class of long acting injectables used to cure chronic ailments such as anti-psychotic or anti-HIV drugs.
pH controlled: These formulations are designed to release the drug after they reach a certain section of the GI track. For example, enteric release tablets are coated with polymers that are insoluble at low pH of the stomach but readily dissolve in the higher pH of the small intestine. These dosage forms are often used to protect the drug from harsh acidic and enzymatic environment of the stomach or to avoid first pass metabolism. This can also enable site specific delivery in the desired part of the GIT and useful in curing local gut diseases.
Osmotically controlled: These systems consist of a tablet core that may have two or more layers. These systems contain drug in different concentrations in top one or more layers and an osmotic agent in the bottom layer. This core is surrounded by a semi-permeable membrane that only allows water to enter but does not allow the drug to diffuse out. When the tablet is exposed to aqueous media, water is diffused into the osmotic layer that swells and pushes out the drug through a laser drilled hole. This type of system has the capability to release medications in unique manners such as at a constant zero order or an ascending release rate.
Gastro-retentive systems: These systems are designed to prolong the residence time of the drug in the stomach. Such systems may be desirable for certain drugs that may have higher stability or absorption characteristics or is required for local action in the stomach. Over the years, several mechanisms of gastro-retention have been developed. Floating systems are low density or effervescent formulations that are driven away from the pyloric valve due to buoyancy in the stomach fluid. High density systems sink below the pylorus and thus stay longer in the stomach. Bio adhesive systems stick to the stomach wall. Swellable and unfolding systems increase in size in the stomach and thus are not easily passed through the pylorus.
Ion-exchange resins: These systems are composed of insoluble polymer drug complexes where the drug release results from exchange of bound drug ions by counterions normally present in body fluids. The drug resin complex is prepared by immersing the ion exchange resins in a concentrated solution of drug. When this complex is administered into the body, it is exposed to counterions that are present in the GI fluid. These counter-ions are exchanged and the drug is released and is finally available for absorption. These systems have numerous applications such as increasing the drug stability, taste-masking and sustained release suspensions.
It is possible to combine two or more mechanistic features in a drug product. Examples include (a) bi- layer tablets with different release mechanisms for each layer, (b) hard gelatin capsules filled with multiple population of beads and (c) CR tablets coated with IR layer to provide an initial burst release.
Development and Manufacturing Considerations
It is important for the product development team to thoroughly understood and consider a range of factors when selecting and designing a CR delivery system. These factors can be broadly classified into three categories:
- Physicochemical and biopharmaceutical properties of the drug such as molecular weight, drug loading, solubility, permeability, stability, partition coefficient, pKa, therapeutic index, elimination half-life and protein binding.
- Human physiology related factors such as patient condition, treatment duration, presence of food, gastric residence and transit times, pH, intra- and inter-subject variability and co-administration of other drugs.
- Target product profile that includes dosage strength, route of administration, desired release profile, target sites, indication, acute or chronic therapy.
A wide range of release controlling polymers are available that can be used depending on the application and route of administration. These include (a) hydrophilic polymers (methycellulose, HPMC, HPC, HEC, Na-CMC, PVP, PEO, etc.), (b) hydrophobic polymers (ethylcellulose, acrylic polymers, HPMC acetate succinate, cellulose acetate, etc.) and (c) natural origin polymers (chitosan, alginates, starch, xanthun gum, carrageenan, gaur gum, pectin, etc.).
Manufacture of controlled release dosage forms usually involves conventional pharmaceutical processes such as direct blending and compression, wet granulation, drying, milling, screening, roller compaction and polymer coating (perforated pan or fluidized bed coating). Because these processes are widely used in the pharmaceutical industry, the quality control and regulatory approval pathways are well established. These factors enable low development cost, rapid timeline and high commercial throughput.
References
Controlled Drug Delivery Systems: Current Status and Future Directions; S Adepu and S Ramakrishna, Molecules, 26 (19); 2021.
Oral Controlled Release Drug Delivery System: An Overview; AA Rao, etal. International Journal of Pharma and Chemical Research. 1 (1), 2015.
Oral Controlled Release Drug Delivery System: An Overview; K Modi etal. International Research Journal of Pharmacy 4(3): 2013.
Oral Controlled Drug Delivery System – A Review; S Jeganath etal. Research Journal of Pharmacy and Technology; 11(2): 2018.






