Vici now offers cGMP API and intermediates synthesis services to support phase 1 clinical and GLP nonclinical studies
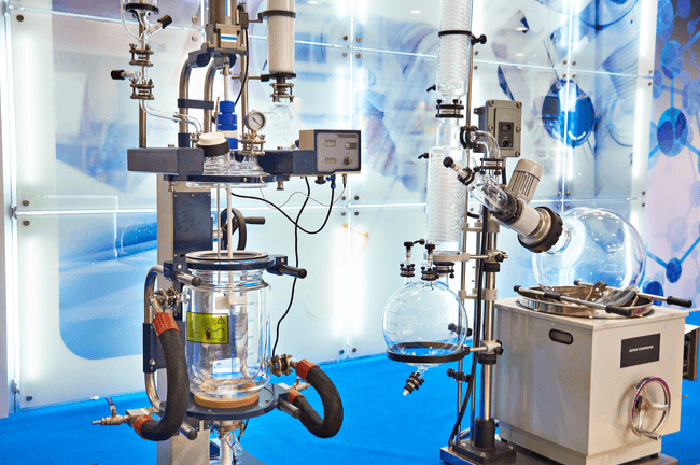
Vici Health Sciences, a Maryland-based CDMO is pleased to introduce cGMP API and API intermediate synthesis services to support phase 1 first-in-human clinical studies and GLP nonclinical studies. This program is built on Vici’s deep expertise in CMC (Chemistry Manufacturing and Controls) ranging from early-phase API and intermediate synthesis through IND enabling cGMP clinical supplies […]
High quality formulation development upfront saves drug developers valuable time and money
Drug development leaders must consider robustness and quality initially during formulation development. In the past, pharmaceutical researchers often did not have a common R&D quality framework for developing robust formulations and manufacturing processes. Consequently, quality was ensured through QC analytical testing. However, in cGMP manufacturing, this leads to expensive batch failure and troubleshooting resulting in […]
The Importance of Formulation Development in Successful Drug Development

Formulation development is a critical part of any drug development program. Once a sponsor selects a new chemical entity (NCE) or 505(b)(2) drug candidate for first-in-human Phase 1 clinic study or a Phase 2 efficacy study, the focus must be on selecting a strong formulation development team to complete a successful investigational new drug (IND) application. Many critical decisions are made […]

