What is Formulation Development?

Pharmaceutical formulation development is the science of developing a dosage form that contains an active ingredient along with inactive ingredients that can be administered to a patient. Examples of dosage forms include oral tablets, oral capsules, oral solutions, oral suspensions, ophthalmic drops, topical semisolids such as creams and ointments, or injections. Why is formulation development […]
What is Content Uniformity and Why is it Important in Oral Solid Dose (OSD) Manufacturing?

Content uniformity (CU) is a quality control measure in pharmaceutical manufacturing that ensures the active pharmaceutical ingredient (API) is evenly distributed within each dosage unit. Content uniformity is a critical to quality attribute (CQA) of a pharmaceutical product that ensures patient safety and drug efficacy. This is particularly important for formulations with very low dosage […]
Orally Disintegrating Tablets (ODTs): A Quick Guide to Fast-Dissolving Medication

Orally disintegrating tablets (ODTs) are solid dosage forms that rapidly dissolve in the mouth without the need for water, typically within seconds to a minute. They are designed to improve patient compliance, especially for those with difficulty swallowing, by providing a convenient and easy-to-administer alternative to traditional tablets. In this article, we’ll explore what ODTs […]
What Is GLP-1: Understanding Its Role in Formulation Development
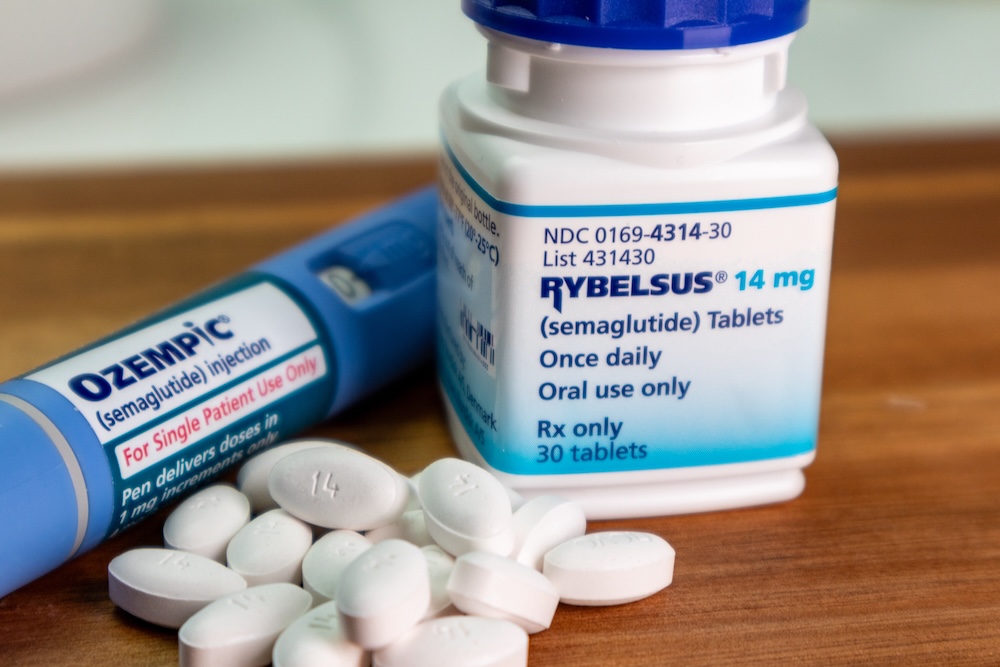
What is GLP-1, and why is it important in formulation development? Glucagon-like peptide-1, or GLP-1, is a hormone that helps regulate blood sugar, manage appetite, and support digestion. Certain medications, known as GLP-1 agonists, mimic these functions and are especially helpful for people with type 2 diabetes or obesity. How can pharmaceutical companies ensure they […]
What is a Compounding Pharmacy and How Can It Help You?

A compounding pharmacy is a specialized type of pharmacy that creates customized medications tailored to meet the unique needs of individual patients. Unlike traditional pharmacies that dispense mass-produced drugs, compounding pharmacies mix ingredients in precise formulations as prescribed by doctors. This personalized approach allows for adjustments in dosage strength, routes of administration, alternative delivery methods, […]
Chewable Tablets: A Guide to Formulation and Development

Chewable tablets are solid oral dosage forms designed to be chewed and swallowed rather than taken whole. They are particularly beneficial for children and those who have difficulty swallowing large tablets. These dosage forms are palatable and conveniently deliver the active ingredients found in vitamins, antibiotics, and analgesics. This guide explores their formulation and development […]
Oral Liquids – A Comprehensive Guide
Oral liquids are an essential part of modern healthcare; they offer patients a convenient way to take their medications. These pharmaceutical formulations disperse active ingredients through a liquid medium and come in various forms, such as suspensions, solutions, and syrups. They are easy to administer, making them an effective alternative for patients who struggle with […]
Taste Masking in Oral liquids
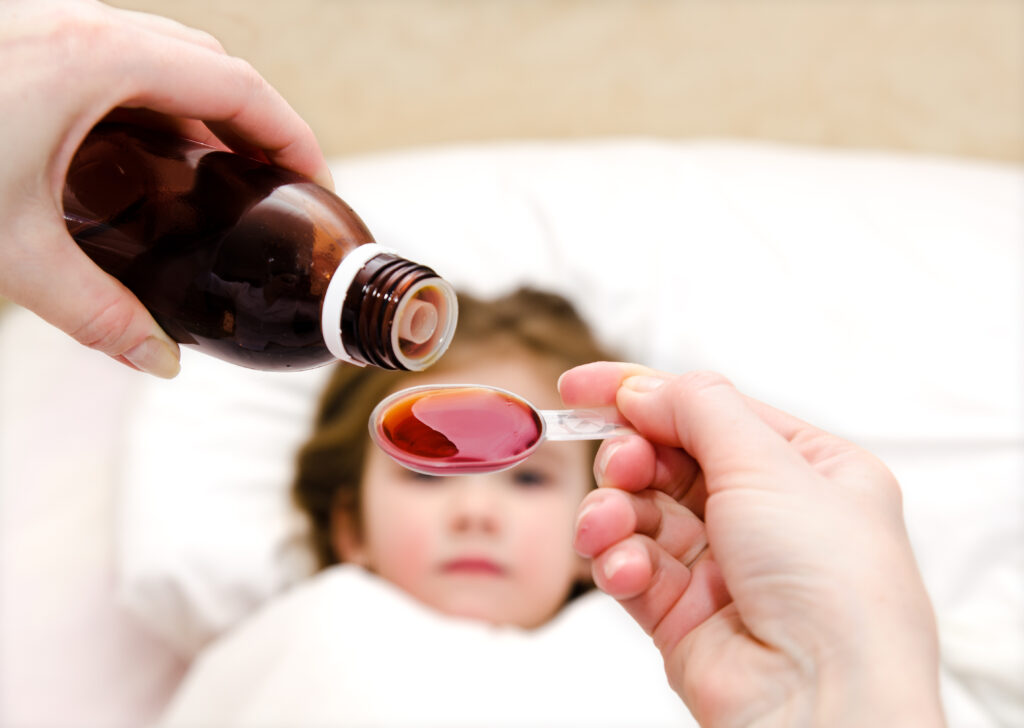
Oral liquids such as syrups and suspensions are the preferred dosage form for pediatric and geriatric patients due to their ease of administration. Swallowing a tablet can be difficult for an infant or an elderly person and injectables usually require a qualified person to administer the drug. Patient compliance in both these drastically decreases if […]
Formulating Fixed-Dose Combination (FDC) Drugs: Innovation through bilayer tablet technology

Bilayer tablets consist of two or more different layers compressed together to form a single tablet. Bilayer tablets are gaining popularity due to their ability to administer multiple active ingredients in one single dosage form. Patients prefer a simple dosing regimen rather than taking multiple different pills throughout the day. Bilayer tablets can help in […]
What is Freeze thaw (Thermal cycling) study?
A freeze-thaw study is a type of stress test performed on liquid formulations to evaluate how finished dosage forms react to repeated cycles of freezing and thawing. For example, when pharmaceutical suspensions formulations or emulsions freeze, they lose their structural characteristics that maintain their properties. Upon thawing, the drug substance may not redisperse properly or may […]

