Analytical Methodology: Validation, Verification, or Qualification – Choosing the Right Approach

Validation, verification, and qualification are often used interchangeably in pharmaceutical testing, but each has their own distinct meanings and all serve different purposes. Getting the test right is just as important in pharmaceutical manufacturing as making the drug. If the analytical method you go with is flawed, every result that follows is questionable. Beyond those […]
Analytical Testing for Peptide Formulations
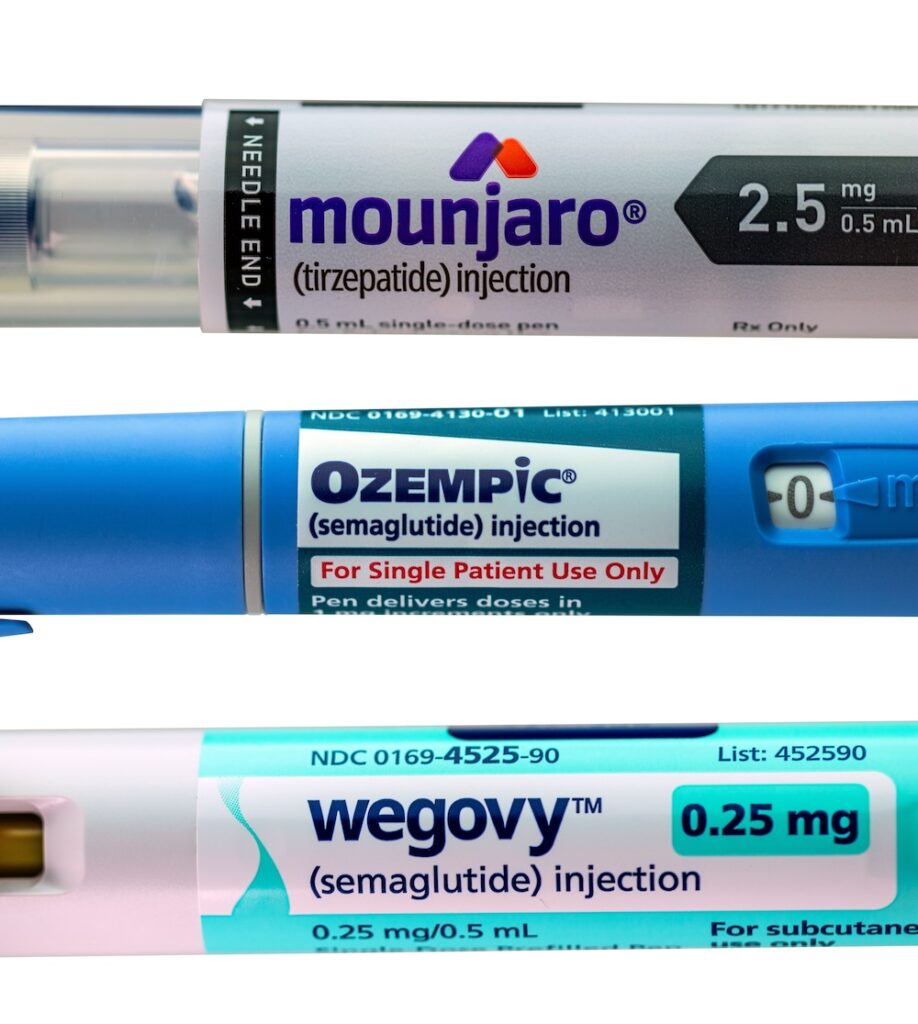
Peptides are commonly used to treat diabetes, obesity, and metabolic disorders. These days, they are also being explored for cancer therapy because they can target specific cells and pathways in the body. Many drugs, including GLP-1 receptor agonists like Semaglutide and Tirzepatide, are examples of peptide-based treatments that have really shown a lot of potential. With […]
What Is HPLC and Why Is It So Commonly Used for Pharmaceutical and Dietary Supplement QC Testing?

HPLC is an advanced sorting tool for QC testing; it allows manufacturers to verify the quality of their products with high precision. It also helps ensure that products comply with cGMP and meet the regulatory requirements set by agencies like the FDA. How Does HPLC Work? HPLC separates substances in a liquid sample based on […]
Is Your Shilajit Authentic? Shilajit Purity Testing

Shilajit is a natural substance found in mountainous regions. It is used in Ayurvedic medicine because it has anti-inflammatory and other potential health benefits. Shilajit contains bioactive compounds such as fulvic acid and humic acid, which contribute to its therapeutic properties. However, the purity of shilajit is crucial for making pharmaceutical products safe and of […]
What is Content Uniformity and Why is it Important in Oral Solid Dose (OSD) Manufacturing?

Content uniformity (CU) is a quality control measure in pharmaceutical manufacturing that ensures the active pharmaceutical ingredient (API) is evenly distributed within each dosage unit. Content uniformity is a critical to quality attribute (CQA) of a pharmaceutical product that ensures patient safety and drug efficacy. This is particularly important for formulations with very low dosage […]
What is Freeze thaw (Thermal cycling) study?
A freeze-thaw study is a type of stress test performed on liquid formulations to evaluate how finished dosage forms react to repeated cycles of freezing and thawing. For example, when pharmaceutical suspensions formulations or emulsions freeze, they lose their structural characteristics that maintain their properties. Upon thawing, the drug substance may not redisperse properly or may […]
What is In-Use Stability?
An in-use stability study is performed to evaluate the chemical, physical, microbiological, and functional integrity of a pharmaceutical product after it has been opened or used but before it expires. This test is used to establish the shelf life and storage condition recommendations for pharmaceutical products after the primary packaging is opened. For example, oral […]
Does your dosage form need to be sterile? Key differences between sterile and non-sterile dosage forms

Whether a dosage form must be sterile prior to administration depends on its route of administration. Parenteral products such as injectable formulations, ophthalmic formulations, otic formulations, and aqueous inhalation products must be sterile prior to use. Oral solid dosage (OSD) and oral liquids, topical, vaginal and anal suppositories on the other hand need not be […]
Stability Testing for Pharmaceutical Drug Products
Establishing shelf life for medicines is a requirement prior to commercializing medicine or initiating clinical trials. Requirements vary for approved pharmaceutical products, compounded medicine, or dietary supplements. Stability testing is performed on pharmaceutical products during drug development and through the life of the product to establish and verify the shelf life of the product. Packaged […]
Frequently Asked Question: Dissolution testing

Dissolution testing of pharmaceutical drug products is one of the most important tools during drug development and for quality assurance during quality control (QC) release testing. This testing is mandatory for all oral solid dose, oral semi solid dose, and oral suspension products. It is not required for drugs that are already in solution such […]

