Services
Home » Our Services

GMP clinical batch manufacturing for Phase I and Phase II trials
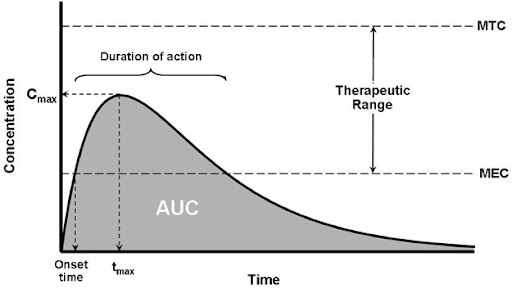
GMP clinical batch manufacturing for Phase I and Phase II trials

HPLC analytical method development, validation, and testing

ICH temperature stability storage and testing

CMO selection, management, tech transfer, and troubleshooting

Pre-IND, IND, ANDA and NDA filing and document support, e-CTD publishing

IP creation and non-infringement strategizing

Consulting services for all stages of product development
GET IN TOUCH
We're Here To Help
We can help you with all your formulation needs. Please call or message us to directly talk to the formulation SME at Vici.

GET A

