IVRT
Home » IVRT
In Vitro Release Testing (IVRT)
In vitro drug release tests are critical for evaluating the product quality of complex drugs, like ophthalmic emulsion drugs.
An in vitro release test (IVRT) can be an important tool to support a demonstration of bioequivalence and/or product quality for various generic drug products. In vitro-in vivo correlation (IVIVC) can allow a prediction of the in vivo performance of a drug based on the IVRT profiles, which can be used to support novel alternative in vitro-based bioequivalence approaches and/or post-approval changes to generic drugs.
Services we offer:
- Method development and validation
- Selection of receptor medium – change in pH of diffusion medium during run
- Membrane selection
- Membrane binding test
- Membrane resistance to free diffusion test
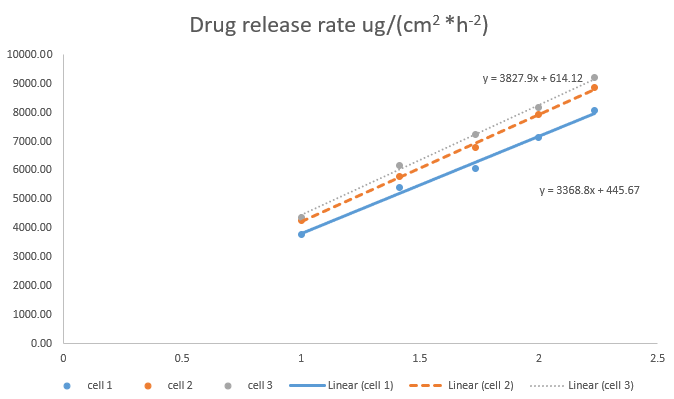
- Determination of in vitro release rate
- Procedural consideration of IVRT method & experimental design – Number of samples, sample size, mass balance, sampling intervals, time course, and spin speed.
- Discriminative ability of the method (such as polymorphic form of API and different grade or source and amount of excipients)
- Product testing to support formulation development and to monitor batches from development, scale-up to commercial manufacturing
- IVRT services to support shelf-life stability, lot variability and site-transfer studies
- Comparative testing of generic formulations with Reference Listed Drug to establish bioequivalence in accordance with Mann-Whitney Rank Sum Test
- Documentation support for submission to regulatory agencies
Topical semisolid dosage forms, which are normally presented in the form of ointments, creams, lotions, and gels, are widely used delivery systems for both systemic therapies delivered through the skin, and therapies that treat the skin itself.

