505(b)(2) Strategy & Consulting
Home » Regulatory Services » 505(b)(2) Strategy & Consulting
505(b)(2) Regulatory Strategy and Consulting
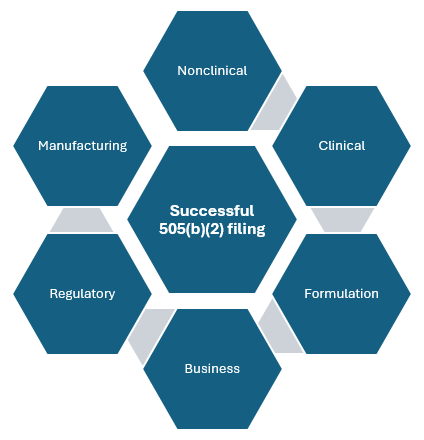
Vici provides NDA 505(b)(2) regulatory strategy, consulting, and FDA filing services. The 505(b)(2) filing pathway allows sponsors to reference existing publicly available safety and effectiveness data from previously approved products. This strategy saves pharmaceutical companies considerable time and money in comparison to the 505(b)(1) pathway. Typically, the NDA 505(b)(2) pathway is used to introduce new pharmaceutical products that use repurposed versions of existing approved drugs for new therapeutic indications or to introduce new dosage forms that enhance patient safety or convenience.
Examples of 505(b)(2) product development efforts at Vici include:
- Change the dosage form from an oral solid dose to an oral liquid formulation to improve swallowability for geriatric patients or patients with dysphagia
- Developing formulations focused on pediatric or infant patients
- Change in the route of administration, such as converting injectable formulations to inhalation or oral drugs
- Developing once-daily extended release or controlled release version of immediate release drugs
- The development of fixed dose combination products referencing single-drug (API) products that are currently marketed
The CMC (chemistry, manufacturing, and controls) requirements for 505(b)(2) products are in general similar to other pharmaceutical products such as ANDA or NDA 505(b)(1) products. However, unlike NCE molecule development, there is likely to be API (active pharmaceutical ingredients) vendors who can supply API DMF (drug master files) that are ready to reference thereby considerably reducing drug substance CMC work requirement.
The extent of nonclinical (animal) toxicology and clinical studies needed will depend significantly upon the available data for referencing. On the lower extreme, product development may need no animal toxicology studies and just a simple pharmacokinetic BA/BE (bioavailability/bioequivalence) study in volunteers. Such programs can be brought to market well under five million dollars including the FDA filing fee and may be completed in three years. On the other hand, some programs may require multi-species animal toxicology studies and phase 1, phase 2, and phase 3 trials thereby costing over a hundred million dollars and may require five or more years to complete.
Formulation patents are an important part of any successful 505(b)(2) go-to-market strategy. This is especially true because the number of years of NCE exclusivity may be as low as zero years. At Vici, we are experts at identifying patent opportunities based on the innovative formulation development work we perform in our US-based company. We have experience working with IP lawyers to create strong patents that will stand the test of time. Reach out to us for your formulation patent needs.
Being a full-service CDMO that can develop formulations, develop and validate analytical methods, manufacture clinical supplies, and file the IND and NDA, Vici can provide all the project leadership and management you need to make your idea reach the market. Talk to our experts to discuss your 505(b)(2) development needs.
505(b)(1) vs 505(b)(2): Understanding the Key Differences…
The 505(b)(1) pathway is typically used for new drugs with…
The FDA’s 505(b)(2) Explained
Pharmaceutical innovation can stem from a variety of different facets…
Contact our management team to learn more about how we can help you with your regulatory filings. We are happy to share our knowledge and provide you with a target budget and timeline for your product.

Ready to Get Started?
Tell us about your upcoming project