Thanks for Visiting Us at SupplySide Connect New Jersey 2025!

Thanks for Visiting Us at SupplySide Connect New Jersey 2025! We had a great time exhibiting at SupplySide Connect and a sincere thank you to everyone who stopped by our booth. It was a pleasure connecting with brands and professionals in the dietary supplements industry and sharing more about how Vici provides end-to-end development solutions including […]
Analytical Testing for Peptide Formulations
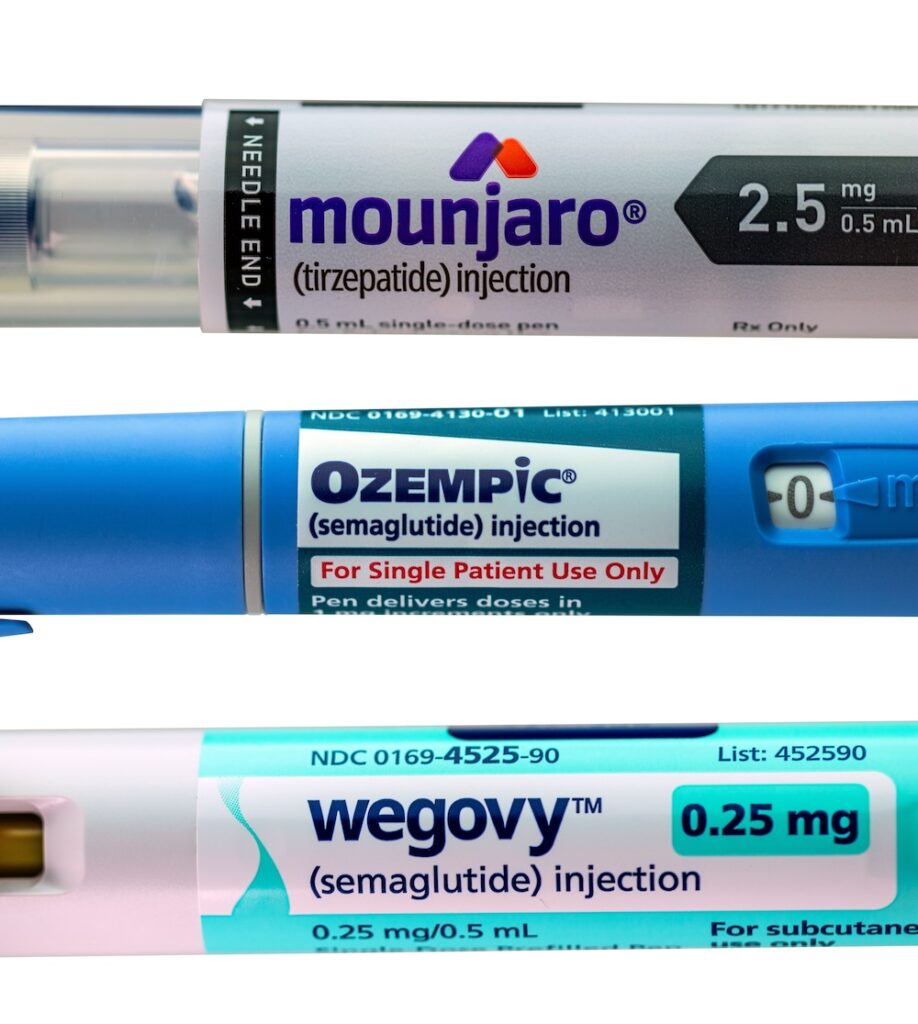
Peptides are commonly used to treat diabetes, obesity, and metabolic disorders. These days, they are also being explored for cancer therapy because they can target specific cells and pathways in the body. Many drugs, including GLP-1 receptor agonists like Semaglutide and Tirzepatide, are examples of peptide-based treatments that have really shown a lot of potential. With […]
What Is HPLC and Why Is It So Commonly Used for Pharmaceutical and Dietary Supplement QC Testing?

HPLC is an advanced sorting tool for QC testing; it allows manufacturers to verify the quality of their products with high precision. It also helps ensure that products comply with cGMP and meet the regulatory requirements set by agencies like the FDA. How Does HPLC Work? HPLC separates substances in a liquid sample based on […]
Regulatory Project Management in Small Pharma: Strategies for Compliance and Success

You will find that in the pharmaceutical industry, regulatory project management is important for making sure pharma companies succeed. Think of it, if you will, as the bridge between innovation and regulatory compliance; without regulatory project management, it would be challenging to meet the requirements of regulatory bodies like the FDA. Effective project management helps […]
Solid State Properties of Drugs

A thorough understanding of the solid state properties of drugs is vital for pharma companies that want to develop effective pharmaceuticals. These properties affect how stable, soluble, and bioavailable a drug will be. In essence, a good grasp of how active pharmaceutical ingredients (APIs) behave in their solid form is important for making effective drugs. […]
Is Your Shilajit Authentic? Shilajit Purity Testing

Shilajit is a natural substance found in mountainous regions. It is used in Ayurvedic medicine because it has anti-inflammatory and other potential health benefits. Shilajit contains bioactive compounds such as fulvic acid and humic acid, which contribute to its therapeutic properties. However, the purity of shilajit is crucial for making pharmaceutical products safe and of […]
What is Formulation Development?

Pharmaceutical formulation development is the science of developing a dosage form that contains an active ingredient along with inactive ingredients that can be administered to a patient. Examples of dosage forms include oral tablets, oral capsules, oral solutions, oral suspensions, ophthalmic drops, topical semisolids such as creams and ointments, or injections. Why is formulation development […]
What is Content Uniformity and Why is it Important in Oral Solid Dose (OSD) Manufacturing?

Content uniformity (CU) is a quality control measure in pharmaceutical manufacturing that ensures the active pharmaceutical ingredient (API) is evenly distributed within each dosage unit. Content uniformity is a critical to quality attribute (CQA) of a pharmaceutical product that ensures patient safety and drug efficacy. This is particularly important for formulations with very low dosage […]
Orally Disintegrating Tablets (ODTs): A Quick Guide to Fast-Dissolving Medication

Orally disintegrating tablets (ODTs) are solid dosage forms that rapidly dissolve in the mouth without the need for water, typically within seconds to a minute. They are designed to improve patient compliance, especially for those with difficulty swallowing, by providing a convenient and easy-to-administer alternative to traditional tablets. In this article, we’ll explore what ODTs […]
What Is GLP-1: Understanding Its Role in Formulation Development
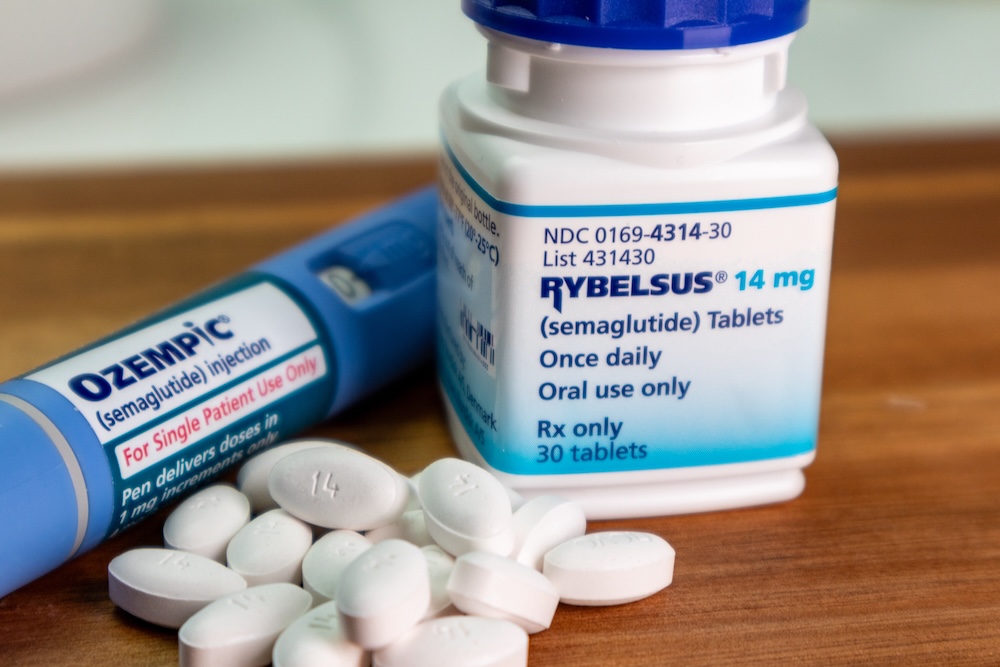
What is GLP-1, and why is it important in formulation development? Glucagon-like peptide-1, or GLP-1, is a hormone that helps regulate blood sugar, manage appetite, and support digestion. Certain medications, known as GLP-1 agonists, mimic these functions and are especially helpful for people with type 2 diabetes or obesity. How can pharmaceutical companies ensure they […]

