Pharmaceutical Suspensions – Part 2: Formulation Development

Early Preformulation Studies For the development of a robust suspension product, several factors must be considered early on during product development. These include API particle size, particle density, viscosity and specific gravity of the media, buffer capacity, hydrophilicity / hydrophobicity, zeta potential, pH-solubility and stability profile of the API, taste masking requirements and preservative efficacy. […]
Vici Health to exhibit at the 22nd Annual Contract Pharma Conference September 21-22, 2023

Vici will be exhibiting at the 22nd Annual Contract Pharma Conference September 21-22, 2023 Hyatt Regency, New Brunswick NJ. As a proud Gold Level Sponsor of the event, Vici invites you to Table 101 to understand your pharmaceutical R&D needs and discuss how we may help grow your drug development pipeline.
Pharmaceutical Suspension Formulations – Part 1: An Overview of Key Advantages and Desirable Properties

What are Pharmaceutical Suspensions Liquid oral dosage forms such as solutions and suspensions are often preferred for pediatric or geriatric patients that find it difficult to swallow tablets or capsules. Most pharmaceutical suspensions are coarse dispersions of solid insoluble drug particles dispersed in a liquid vehicle. Besides oral administration, suspensions are also prepared for other […]
Buccal Delivery Systems (Part 3) – Improving Bioavailability through Buccal Route
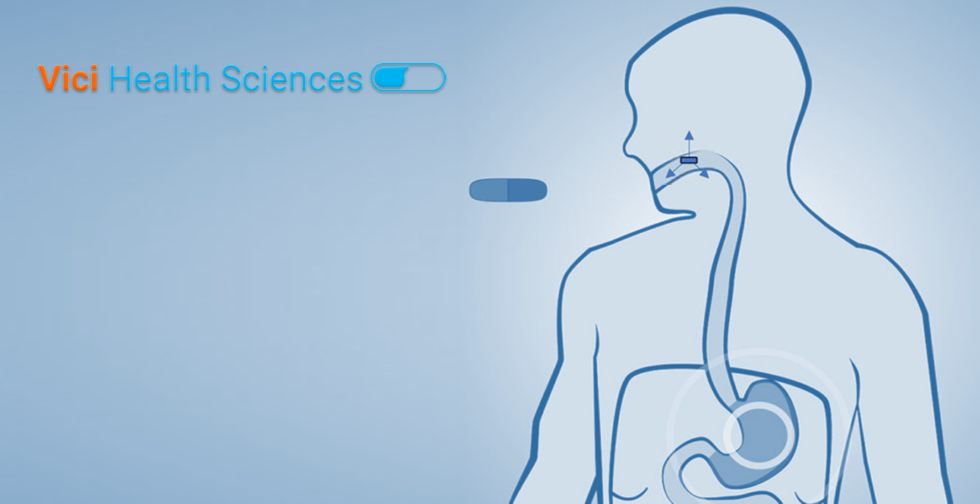
Poor bioavailability is a common problem for a majority of new drugs in the pipeline of pharmaceutical companies. This can be due to a variety of reasons including poor solubility, permeability, susceptibility to low pH environment, enzymatic degradation or hepatic first pass metabolism. An estimated 70-80 % of pipeline drugs under development fall under BCS […]
Vici offers phase 1, phase 2, and phase 3 clinical supplies manufacturing services

Through strong partnerships with our sister manufacturing company, Vici is now able to provide Phase 3 clinical manufacturing services to support NDA programs. With this offering, Vici is able to offer a full range of integrated CDMO services starting from early phase cGMP API synthesis to support nonclinical toxicology and proof-of-concept studies, first-in-human formulation development […]
Buccal Delivery Systems | Part 2: Applications and Dosage Forms
Why Buccal Delivery Buccal cavity in the mouth is an attractive route for administration of drugs that offers numerous benefits such as rapid product development timelines, reduced cost of therapy, ease of administration and improved adherence to therapy. It is especially useful for administration of drugs that undergo extensive first pass metabolism. Increased bioavailability can […]
Vici is excited to announce that Dr Suneel Rastogi will be joining our technical leadership team.

Dr. Rastogi has over 24 years of extensive experience in the development of a variety of conventional and complex dosage forms including oral dosage forms, injectables, long acting, amorphous solid dispersions, ophthalmic, topical, nano particulates, peptides, nasal sprays and rectal gels. He has a proven track record of successfully managing over 100 internal and external […]
Buccal Delivery Systems | Part 1: Opportunities and Challenges
The buccal region is the mouth cavity offers unique benefits for delivery of drugs over conventional more popular routes of administration such as oral, parenteral and topical. Buccal mucosa is highly perfused with blood vessels which enables rapid absorption and improved bioavailability of many drugs for both local and systemic treatments. It combines the beneficial […]
The science of pharmaceutical taste masking

Taste masking is often used in the pharmaceutical industry to make bitter or unpleasant-tasting drugs more palatable. This is especially important for pediatric populations or geriatric populations. Taste masking can be achieved through a variety of methods, including: Adding sweeteners and flavors: Sweeteners and flavors can be added to mask the taste of a drug. […]
Vici Health exhibiting at the CPHI North America Conference in Philadelphia, Pennsylvania April 25-27, 2023

Vici Health, a world class pharmaceutical product development company, announced that it will be exhibiting at the CPHI North America Conference in Philadelphia, Pennsylvania April 25-27, 2023. Based in Maryland, USA, Vici provides CDMO services that include API synthesis, formulation development, analytical development and testing, phase 1 and 2 cGMP clinical supplies manufacturing, and Regulatory/CMC […]

