SBIR Grants for Early-Stage Drug Development

Small Business Innovation Research (SBIR) grants for early-stage drug development offer start-up biopharma companies seed funding that helps turn their innovative drug therapy concepts into successful FDA drug development programs. Bringing a new drug to the market can be quite complex and expensive, especially for small pharmaceutical companies. That is where the SBIR and Small […]
Does your dosage form need to be sterile? Key differences between sterile and non-sterile dosage forms

Whether a dosage form must be sterile prior to administration depends on its route of administration. Parenteral products such as injectable formulations, ophthalmic formulations, otic formulations, and aqueous inhalation products must be sterile prior to use. Oral solid dosage (OSD) and oral liquids, topical, vaginal and anal suppositories on the other hand need not be […]
Overcoming Key Challenges in Developing Fixed-Dose Combination Tablets

Fixed dose combination products are dosage forms containing two or more active pharmaceutical ingredients. Including more than one active ingredient in the same dosage form has shown to increase the effectiveness of treatment for infective diseases such as HIV and tuberculosis and also chronic conditions such as blood pressure and diabetes, where patient compliance is […]
Why CMC Experts Are Crucial for Successful Drug Development

The acronym CMC stands for Chemistry, Manufacturing, and Controls and is widely used in the pharmaceutical industry and by regulatory bodies including the US FDA. The discipline of CMC includes formulation development, drug substance and drug product manufacturing, pharmaceutical analytical testing, and the overall control strategy used to ensure quality and regulatory compliance of pharmaceutical […]
Stability Testing for Pharmaceutical Drug Products
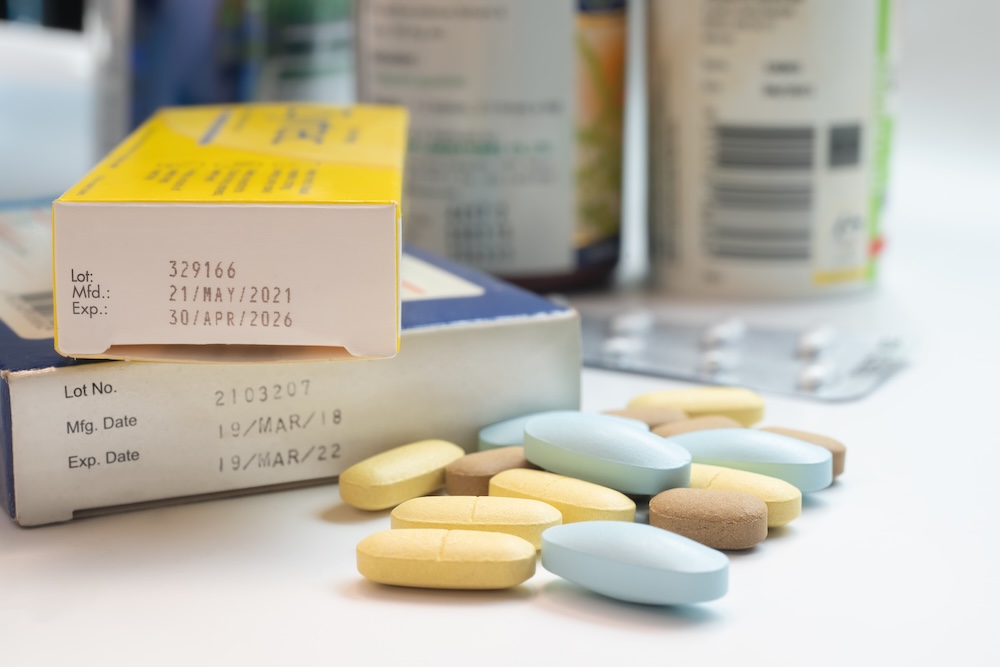
Establishing shelf life for medicines is a requirement prior to commercializing medicine or initiating clinical trials. Requirements vary for approved pharmaceutical products, compounded medicine, or dietary supplements. Stability testing is performed on pharmaceutical products during drug development and through the life of the product to establish and verify the shelf life of the product. Packaged […]
What is an IND?
An IND is an Investigational New Drug Application that is required by a clinical study sponsor to obtain authorization from the Food and Drug Administration (FDA) to perform human clinical studies for investigational drug or biological product. This is federally mandated, and the requirements are set forth in 21 CFR Part 312. Why are INDs […]
Patient Compliance Through Drug Delivery Systems

Lack of patient compliance in medication is a major cause of poor health outcomes. There are a variety of reasons that contribute to this lack of patient compliance. Forgetfulness, adverse side effects, lack of access to medicine, high cost, inability to swallow large tablets or capsules, fear of needles, and poor taste are all reasons […]
What is CMC?

In the pharmaceutical industry, CMC stands for Chemistry Manufacturing and Controls. Quality and consistency of medicines is the primary responsibility and focus of the CMC function. The FDA regulates all CMC requirements for the pharmaceutical industry, which covers all aspects of drug substance and drug product chemistry, manufacturing, and quality control (QC). CMC consists of […]
Frequently Asked Question: Dissolution testing

Dissolution testing of pharmaceutical drug products is one of the most important tools during drug development and for quality assurance during quality control (QC) release testing. This testing is mandatory for all oral solid dose, oral semi solid dose, and oral suspension products. It is not required for drugs that are already in solution such […]
Why Your Early-Stage Drug Development Needs an R&D-Focused CDMO Partner

Early-stage drug development is best performed at an R&D-focused CDMO. Initial formulation and small batch manufacturing for proof-of-concept clinical trials require speed, agility, and cost-effectiveness as opposed to the scale of a Phase III or routine commercial manufacturing-focused CMO. Preclinical work in early-stage drug development encompasses the following: Drug discovery Drug substance characterization Early-stage […]

