Pharmaceutical Dissolution Testing
Home » Analytical Testing » Dissolution Testing
Dissolution Testing
Dissolution testing is required for all solid oral dosage forms and oral suspensions. Tablet, capsule, suspensions, emulsions, and any dosage form where the drug substance is not dissolved must undergo USP dissolution testing both during R&D development and for quality control (QC) purposes. Vici Health Sciences not only has experience developing and performing R&D and cGMP QC dissolution testing for clients, but we also have the depth of formulation and regulatory knowledge to troubleshoot problems that arise due to out-of-specification (OOS) data. You have full access to our pharmaceutical science experts who can guide you through challenging OOS investigations, ensuring your product reaches the clinic or market quickly and stays there.

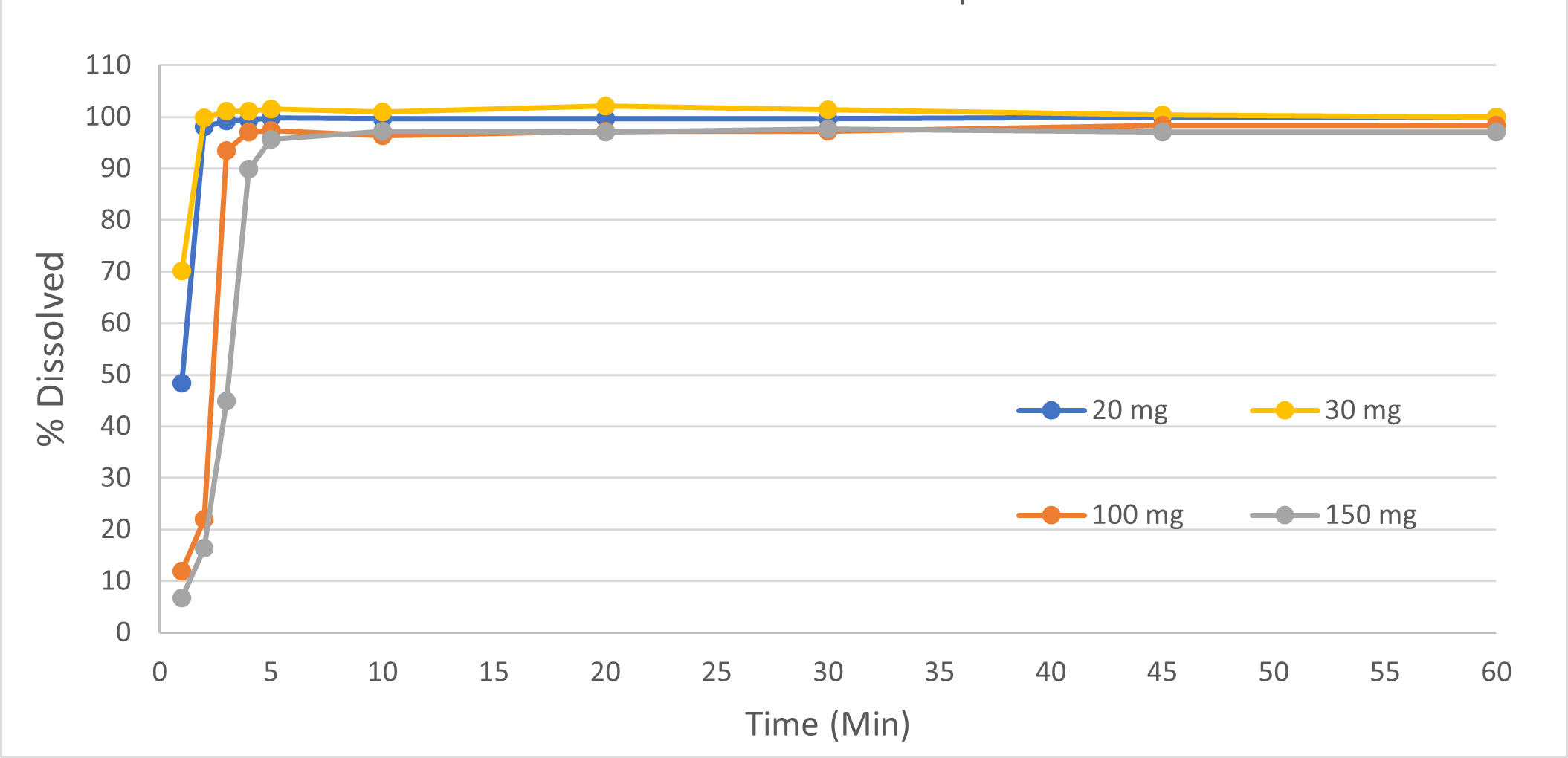
Dissolution Testing for QC Release and Stability
Regulatory agencies expect drug developers and commercial drug manufacturers to perform QC release through dissolution testing for tablets, capsules, suspensions and other dosage forms where the drug is not dissolved.
Vici performs dissolution using USP 1 or USP 2 equipment and has state-of-the-art Vanke™ and Distek™ dissolution apparatuses equipped with autosamplers suitable for performing dissolution testing on immediate-release, enteric, and extended-release products. Our equipment undergoes regular calibration and preventive maintenance per cGMP requirements to ensure that results are accurate, reliable, and consistent.
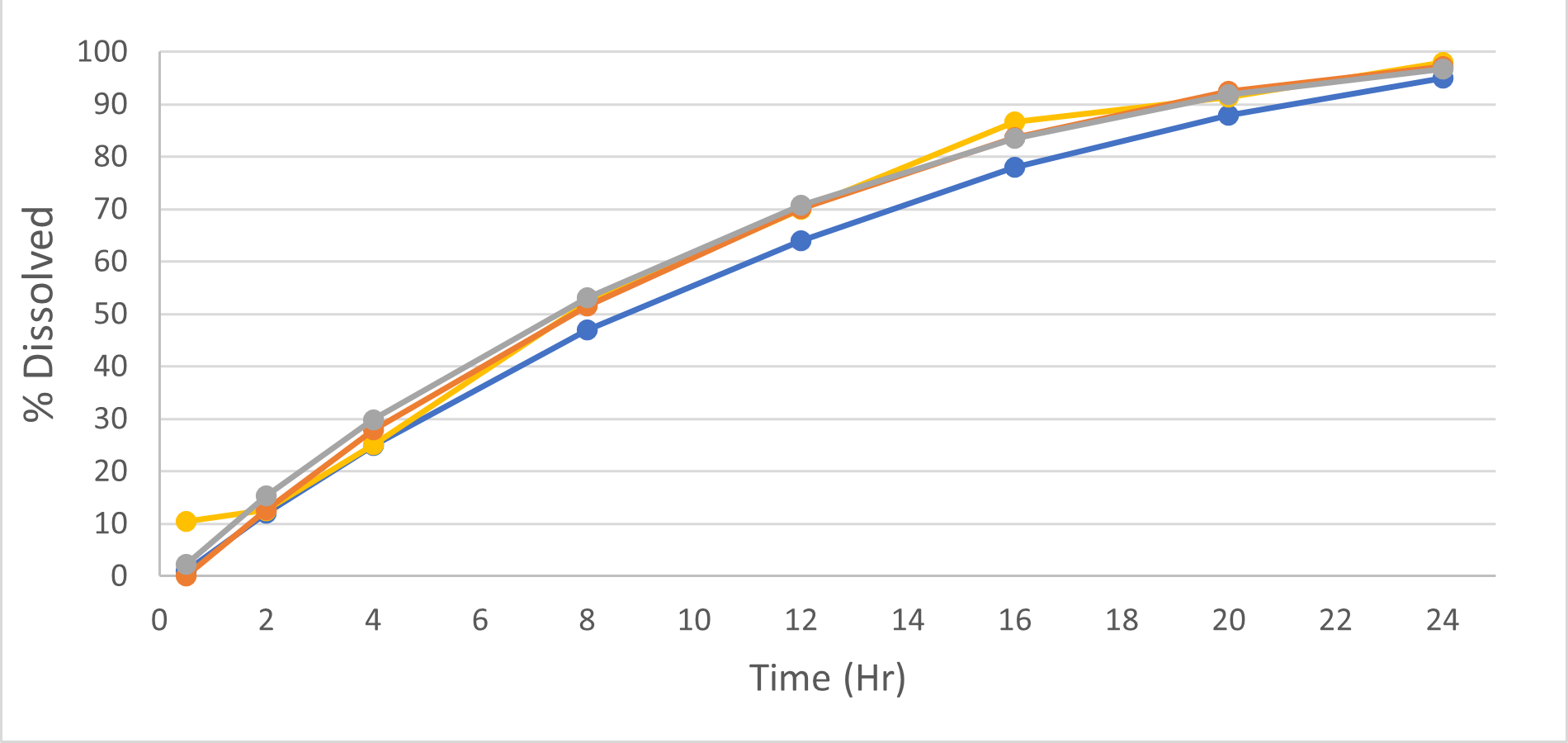
Dissolution Testing for Product Development (R&D)
We view dissolution testing as a critical component of pre-clinical drug development and a valuable tool for ultimate clinical success.
We are experienced in the development and utilization of discriminatory dissolution methodologies, notably for BCS-2 and BCS-4 products. Such methodologies, when applied correctly, can help ensure bioequivalence to reference products, needed for generic (ANDA), NDA 505(b)(2), and even NDA 505(b)(1) product development.
Vici can develop and utilize biorelevant dissolution methodologies using biorelevant media to mimic drug release in the stomach or intestine or even small volume dissolution testing to mimic drug release in the mouth. Such techniques, when used in conjunction with knowledge of biopharmaceutics and formulation science, can help speed up drug development and save money in the clinic during Phase I and Phase II clinical trials.
We're Here to Help
Our industry experts at Vici Health Sciences can bring your product to market faster.
Schedule a free consultation for your project with one of our experts.

