All medicine administered to patients must first be formulated. It’s not possible to provide pure active pharmaceutical ingredients (API) to patients as treatment. The drug must first be formulated into a stable, manufacturable dosage form which releases the correct dose of active ingredient at a precise site of action to be of efficacy. All while upholding the integrity to patient safety by limiting unwanted toxic side effects. This requires formulation development and optimization to be a part of each phase throughout drug development and commercial manufacturing.
The Stages of Drug Development
The lifecycle of a pharmaceutical drug product is generally classified into four sections:
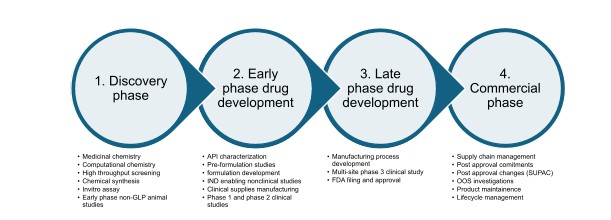
After the initial discovery phase, formulation development plays a central role in drug development and manufacturing. Early-stage preclinical work includes pre-formulation or physicochemical characterization of the drug substance or API. This characterization includes solubility screening, salt screening, solid-state characterization, and evaluation of stability of the drug molecule. Drugs with poor solubility and stability require special formulation techniques to ensure that the drug molecule is bioavailable and reaches the desired site of action.
Route of Administration and Formulation Design
Once the drug substance is characterized and the site of action has been identified, the route of administration must be determined. Possible routes of administration include:
| Route of Administration | Site of Entry |
| Oral | Drug delivery to the buccal cavity, stomach, intestines, or colon |
| Injectable | Can be subcutaneous, intramuscular, or intravenous |
| Topical or Transdermal | Applied to the skin to target local or systemic sites of action |
| Inhalation | Devices delivers drug to the lungs |
| Nasal | Spray drug delivery in the nose |
| Ophthalmic | Drug delivery through the eye |
| Otic | Drug delivery through the ear canal |
| Suppository | Drug delivery through the rectum |
| Vaginal | Drug delivery through the vagina |
Upon finalizing the route of administration, the desired pharmacokinetic profile is determined, either through clinical expertise and medical experience or through pharmacokinetic and pharmacodynamic modeling.
Drug Delivery Systems
The next step in the formulation development process is to develop a dosage form that releases the drug at the right rate and location within the body. The dosage form can be formulated as an immediate release or controlled release product.
Immediate release formulations for highly soluble drugs are relatively simple to develop. Drugs with poor bioavailability or very poor stability will often require more complex drug delivery systems.
Vici has experience working on a variety of the different drug delivery systems in the market including extended-release matrix tablets, osmotic tablets for controlled release, pH sensitive polymer coated enteric tablets, coated multiparticulate bead filled capsules for controlled release, and self-emulsifying drug delivery systems (SEDDS) to enhance bioavailability and bypass first pass liver metabolism.
Formulation Development to Support Clinical Studies
Once the drug specifications have been established, clinical trial material is then formulated to support early phase, such as Phase I and Phase IIa, clinical studies. Early phase clinical trial manufacturing is often supported closely by the formulation development team as the manufacturing process is not finalized and the product is still under development. As the program progresses into the later Phase IIb and Phase III stages, the formulation undergoes further final optimizations to be ready for commercial launch.
Formulation Development and Pharmaceutical Regulatory Compliance
The formulation development team plays another pivotal role in ensuring Chemistry, Manufacturing and Controls (CMC) regulatory compliance, critical for successful IND and NDA filing. Using the correct ingredient grade and level selection, setting the right specifications, choosing the appropriate packaging components selection, and generating sufficient stability data on the dosage form are some of the critical activities necessary to ensure a smooth CMC regulatory pathway for the product.
Analytical Methods and Product Specifications
Specifications are set for all critical quality attributes of the drug product including product ID, assay, impurity levels, uniformity of dosage form, residual organic solvents, heavy metals, nitrosamine impurities, and a variety of process measurements for quality control during manufacturing. Dosage form-specific specifications such as API particle size, viscosity, osmolarity, pH, viscosity, particulate matter, preservative assay, preservative efficacy, and microbial content are also considered.
Manufacturing Process Optimization and Scale Up
Both formulation and manufacturing influence the properties of the final dosage product and effective formulation development cannot be disconnected from manufacturing process optimization and scale-up. Formulation scientists often oversee operations related to manufacturing process scale-up to ensure the fundamental properties of the formulation remain unchanged as modifications are made to ensure ease of manufacturing and robustness. The formulation development team also provides all the CMC documents needed for FDA filing and responds to queries that arise during the review process.
Supply Chain Management and Post Approval Changes
The need for R&D support, especially from the formulation development team, does not end upon receiving FDA approval. Pharmaceutical supply chains involve a complicated variety of raw materials with testing vendors spanning the globe. Raw material source changes or specifications are not uncommon, which can affect product performance. From a regulatory perspective, pharmaceutical product guidelines are constantly updated by the FDA and other global regulatory agencies. Manufacturing location may also need to be sporadically changed during the commercialization phase of the product. These scenarios require the oversight of an experienced formulation development team that can stay agile in unforeseen circumstances to successfully manufacture an approved product within the framework of SUPAC guidance and keeping it in the market.
The Vici Advantage
As a company specializing in early-stage formulation development, Vici has a proven track record of delivering formulation products from development to post-approval commercialization. The technical team at Vici oversees dozens of manufacturing process scale-up operations and are experts at anticipating and troubleshooting the technical and regulatory obstacles that invariably occur during the drug development process. To learn more about how Vici can accelerate your product development, send us a contact form or call us at 410-379-1500.






